Abstract
In B lymphoblastic leukemia/lymphoma (B-ALL/LBL), t(9;22)(q34;q11.2) and t(1;19)(q23;p13.3) are recurrent cytogenetic abnormalities. The concurrent occurrence of both abnormalities is very rare, and only 3 cases have been previously reported. Here, we report a case of adult B-ALL with ider(9)(q10)t(9;22)(q34;q11.2) and der(19)t(1;19)(q23;p13.3). A literature review revealed that ider(9) (q10)t(9;22) is a rare variant of t(9;22) with a deletion of the short arm of chromosome 9. Fifteen cases of ider(9)(q10)t(9;22) have been reported. This abnormality is specific to precursor B-lymphoid neoplasms, such as B-ALL or B-lymphoid blast phase of CML, and is associated with disease progression or short survival. The cytogenetic abnormality t(1;19) is also specific to B-ALL. In most instances of t(1;19), TCF3 is fused to PBX1; however, a few cases have identical translocations but no TCF3-PBX1 fusion, as was observed in our patient. We describe the first case of ider(9)(q10)t(9;22) in combination with TCF3-PBX1 negative t(1;19). The patient underwent imatinib therapy in addition to intensive chemotherapy, but failed to achieve remission.
Go to : 
REFERENCES
1.Borowitz MJ., Chan JKC. B-lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities. Swerdlow SH, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed.Lyon: International Agency for Research on Cancer;2008. p. 171–5.
2.Mitelman F., Johansson B., Mertens F. Mitelman database of chromosome aberrations and gene fusions in cancer. 2010. http://cgap.nci.nih.gov/Chromosomes/Mitelman. (Updated on Feb 2010).
3.Sessarego M., Defferrari R., Fugazza G., Comelli A., Salvidio E., Ajmar F. Involvement of the short arm of the derivative chromosome 9 in Philadelphia-positive acute lymphoblastic leukemia. Cancer Genet Cytogenet. 1991. 52:43–9.

4.Heerema NA., Palmer CG., Weetman R., Bertolone S. Cytogenetic analysis in relapsed childhood acute lymphoblastic leukemia. Leukemia. 1992. 6:185–92.
5.Dierlamm J., Michaux L., Kröger N., Wlodarska I., Martiat P., Zeller W, et al. ider(9)(q10)t(9;22)(q34;q11) is a recurrent chromosomal abnormality in acute lymphoblastic leukemia and lymphatic blastic phase of chronic myelogenous leukemia. Cancer Genet Cytogenet. 1996. 89:109–13.

6.Rieder H., Ludwig WD., Gassmann W., Maurer J., Janssen JW., Gokbuget N, et al. Prognostic significance of additional chromosome abnormalities in adult patients with Philadelphia chromosome positive acute lymphoblastic leukaemia. Br J Haematol. 1996. 95:678–91.

7.Uckun FM., Nachman JB., Sather HN., Sensel MG., Kraft P., Steinherz PG, et al. Clinical significance of Philadelphia chromosome positive pediatric acute lymphoblastic leukemia in the context of contemporary intensive therapies: a report from the Children's Cancer Group. Cancer. 1998. 83:2030–9.
8.Kansal R., Deeb G., Barcos M., Wetzler M., Brecher ML., Block AW, et al. Precursor B lymphoblastic leukemia with surface light chain immunoglobulin restriction: a report of 15 patients. Am J Clin Pathol. 2004. 121:512–25.
9.Preiss BS., Kerndrup GB., Pedersen RK., Hasle H., Pallisgaard N. Contribution of multiparameter genetic analysis to the detection of genetic alterations in hematologic neoplasia. An evaluation of combining G-band analysis, spectral karyotyping, and multiplex reverse-transcription polymerase chain reaction (multiplex RT-PCR). Cancer Genet Cytogenet. 2006. 165:1–8.

10.Huh J., Chung W. A case of acute lymphoblastic leukemia with ider(9)(q10)t(9;22)(q34;q11.2). Korean J Lab Med. 2006. 26:223–6.

11.Diez-Martin JL., Dewald GW., Pierre RV. Possible cytogenetic distinction between lymphoid and myeloid blast crisis in chronic granulocytic leukemia. Am J Hematol. 1988. 27:194–203.

12.Chan LC., Kwong YL., Liu HW., Chan TK., Todd D., Ching LM. Cytogenetic analysis of hematologic malignancies in Hong Kong. A study of 98 cases. Cancer Genet Cytogenet. 1992. 62:154–9.
13.Anastasi J., Feng J., Le Beau MM., Larson RA., Rowley JD., Vardiman JW. The relationship between secondary chromosomal abnormalities and blast transformation in chronic myelogenous leukemia. Leukemia. 1995. 9:628–33.
14.Choi GJ., Jeon DS., Chun HJ., Kim JR., Song HS., Lee JW. ider(9)(q10)t(9;22)(q34;q11.2) as secondary karyotypic aberration of chronic myelogeous leukemia. Korean J Clin Pathol. 1999. 19:266–70.
15.Shearer BM., Flynn HC., Knudson RA., Ketterling RP. Interphase FISH to detect PBX1/E2A fusion resulting from the der(19)t(1;19) (q23;p13.3) or t(1;19)(q23;p13.3) in paediatric patients with acute lymphoblastic leukaemia. Br J Haematol. 2005. 129:45–52.
16.Shaffer LG., Slovak ML, et al. eds. ISCN. 2009. an international system for human cytogenetic nomenclature (2009). Basel: Karger,. 2009:59–83.
17.Yanada M., Takeuchi J., Sugiura I., Akiyama H., Usui N., Yagasaki F, et al. Karyotype at diagnosis is the major prognostic factor predicting relapse-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Haematologica. 2008. 93:287–90.

18.Faderl S., Estrov Z., Kantarjian HM., Thomas D., Cortes J., Manshouri T, et al. The incidence of chromosome 9p21 abnormalities and deletions of tumor suppressor genes p15INK4b/p16INK4a/p14ARF in patients with acute lymphoblastic leukemia. Cytokines Cell Mol Ther. 1999. 5:159–63.
19.Bertin R., Acquaviva C., Mirebeau D., Guidal-Giroux C., Vilmer E., Cave H. CDKN2A, CDKN2B, and MTAP gene dosage permits precise characterization of mono- and bi-allelic 9p21 deletions in childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2003. 37:44–57.
20.Nowak NJ., Sait SN., Zeidan A., Deeb G., Gaile D., Liu S, et al. Recurrent deletion of 9q34 in adult normal karyotype precursor B-cell acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2010. 199:15–20.

21.Barber KE., Harrison CJ., Broadfield ZJ., Stewart AR., Wright SL., Martineau M, et al. Molecular cytogenetic characterization of TCF3 (E2-A)/19p13.3 rearrangements in B-cell precursor acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2007. 46:478–86.
22.Boomer T., Varella-Garcia M., McGavran L., Meltesen L., Olsen AS., Hunger SP. Detection of E2A translocations in leukemias via fluorescence in situ hybridization. Leukemia. 2001. 15:95–102.
23.Pui CH., Raimondi SC., Hancock ML., Rivera GK., Ribeiro RC., Mahmoud HH, et al. Immunologic, cytogenetic, and clinical characterization of childhood acute lymphoblastic leukemia with the t(1;19) (q23;p13) or its derivative. J Clin Oncol. 1994. 12:2601–6.
24.Griffin TC., Tomlinson GE., Raimondi SC., Sandoval C., Timmons CF., Rosenfield C, et al. Childhood acute lymphoblastic leukemia with both t(1;19) and t(9;22). Leukemia. 1992. 6:535–40.
Go to : 
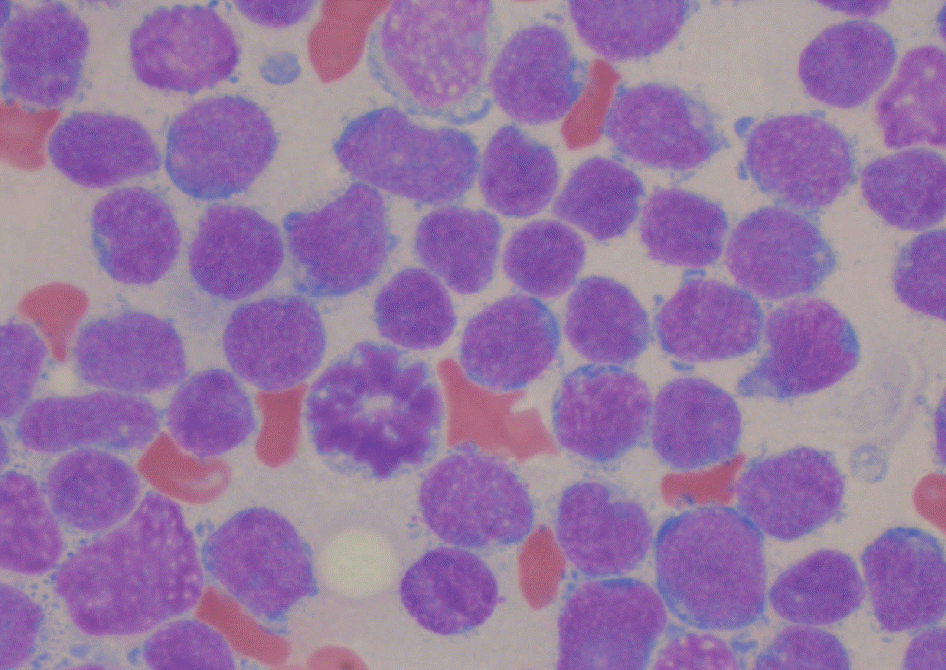 | Fig. 1.The bone marrow aspirates showed an increased number of small sized blasts with scanty cytoplasm and inconspicuous nucleoli (Wright stain, ×1,000). |
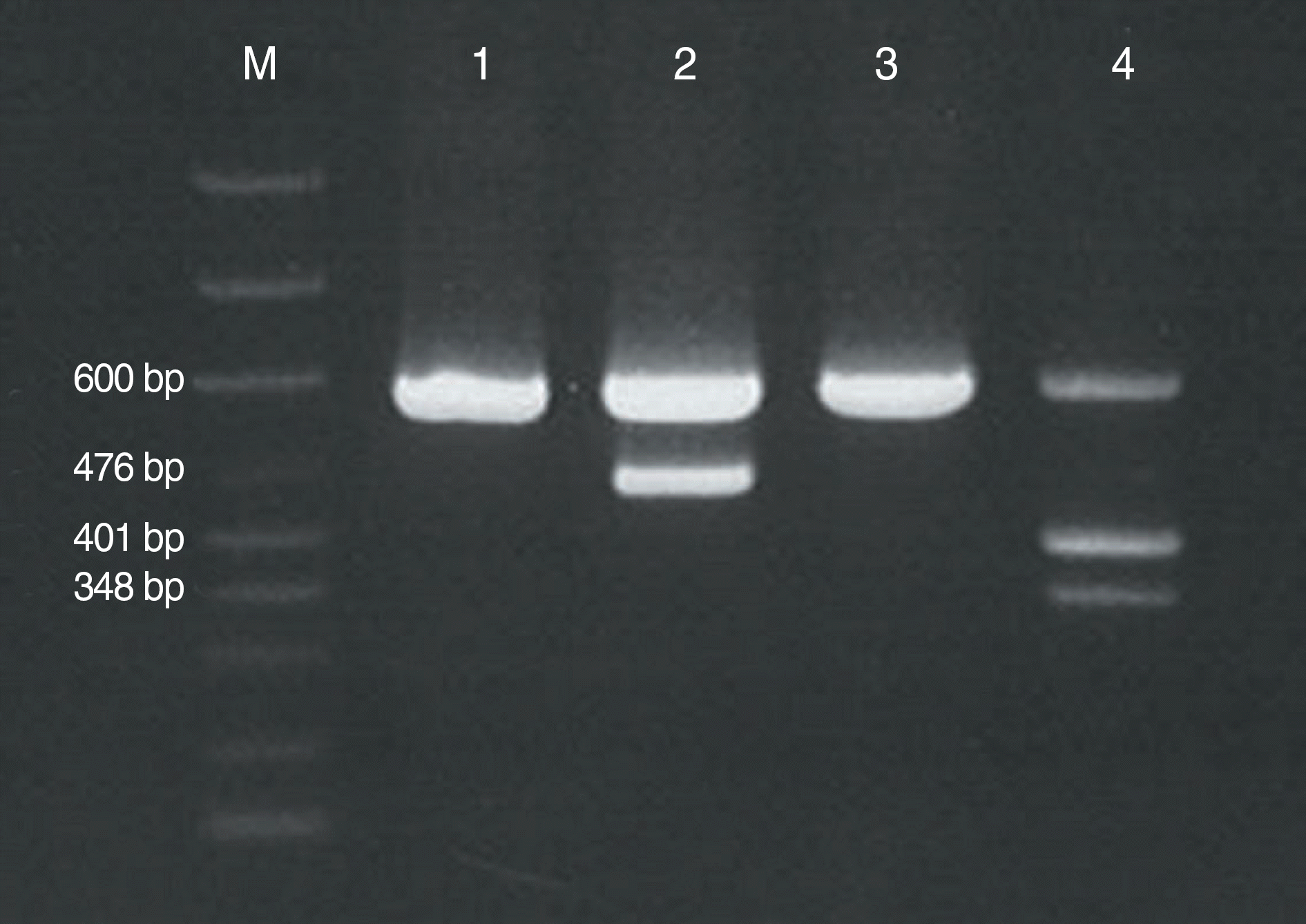 | Fig. 2.Reverse transcriptase PCR demonstrated major BCR-ABL1 gene rearrangement (b3a2) [Lane M, size marker (includes markers for the 600-bp internal control, 476-bp b3a2 fusion transcript, 401-bp b2a2 fusion transcript, and 348-bp e1a2 fusion transcript); lane 1 and 3, negative patients; lane 2, our patient; lane 4, positive control with b2a2 and e1a2 fusion transcripts, provided by the manufacturer]. |
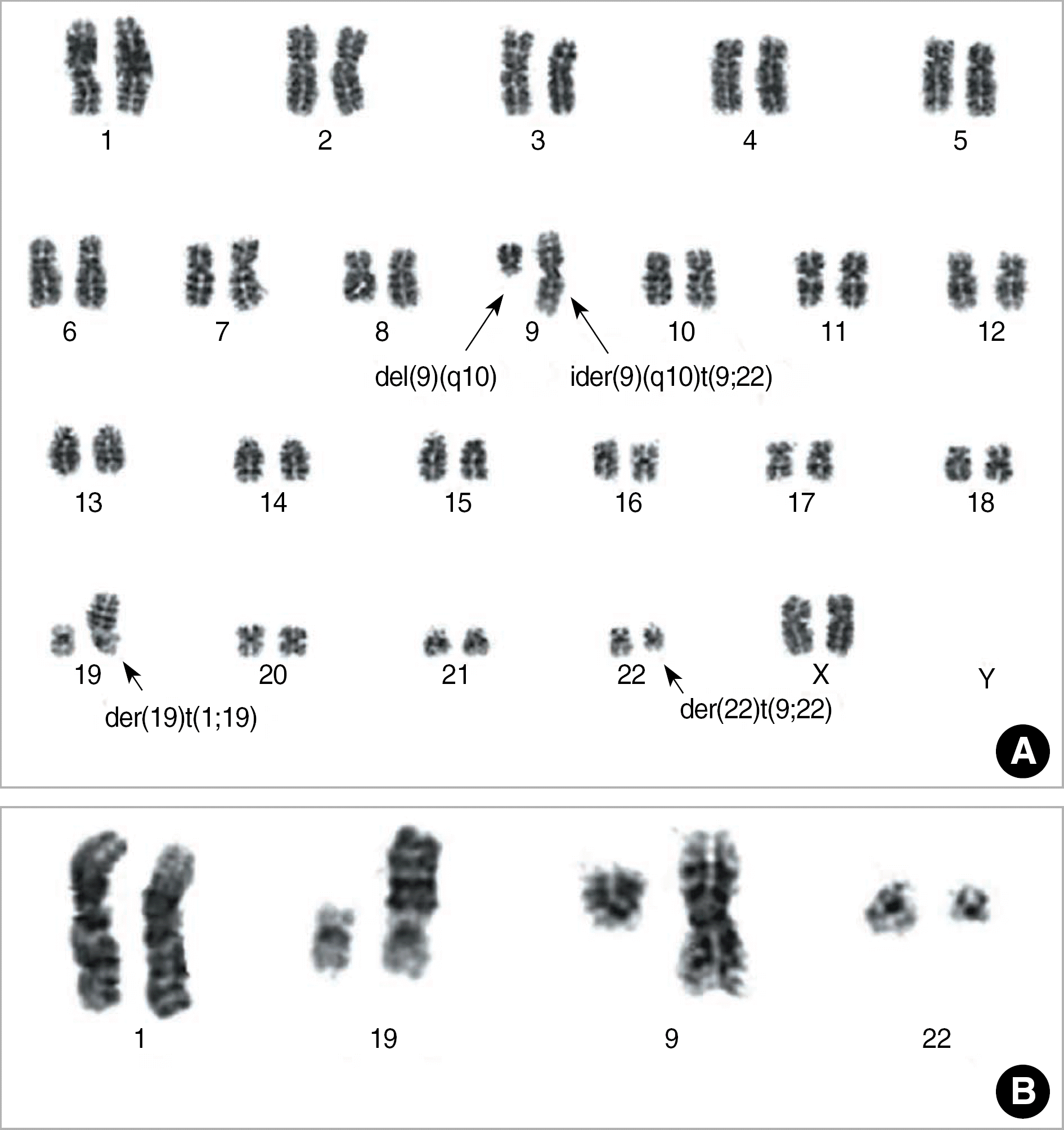 | Fig. 3.The representative full karyogram (A) and partial karyogram (B) showed 46,XX,ider(9)(q10)t(9;22)(q34;q11.2),del(9)(q10),der(19)t(1;19)(q23;p13.3),der(22)t(9;22). |
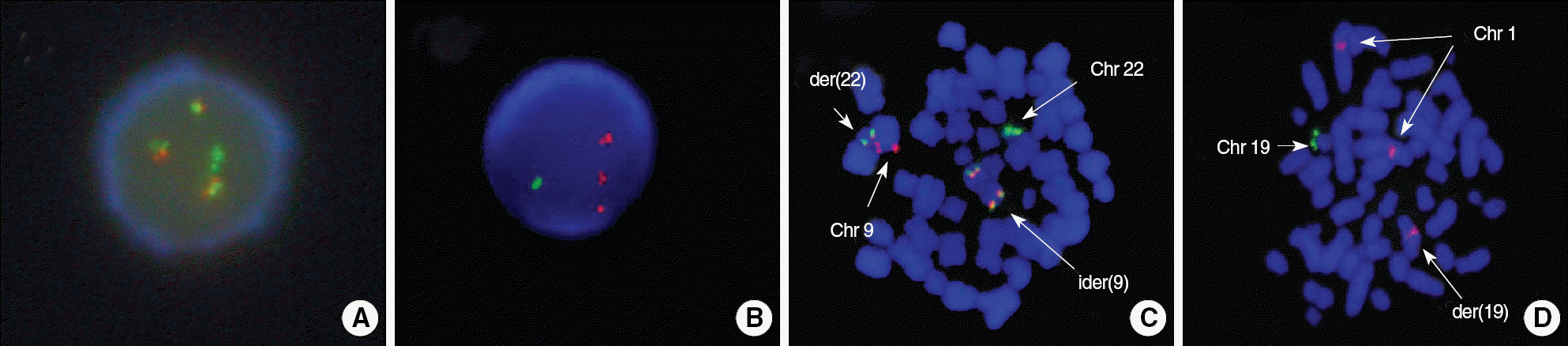 | Fig. 4.(A) FISH using the BCR/ABL1 dual color dual fusion translocation probe at relapse. One green signal represents the normal chromosome 22, and 3 fusion signals are seen. Loss of red signal is consistent with deletion of the long arm of normal chromosome 9. (B) FISH using the TCF3/PBX1 dual color dual fusion translocation probe at relapse showed 1 green and 3 red signals with no fusion signal. (C) FISH using the BCR/ABL1 dual color dual fusion translocation probe at initial diagnosis. Fusion signals are demonstrated on der(22) and both arms of ider(9). (D) FISH using the TCF3/PBX1 dual color dual fusion translocation probe at initial diagnosis. Fusion signal is not observed on der(19). |
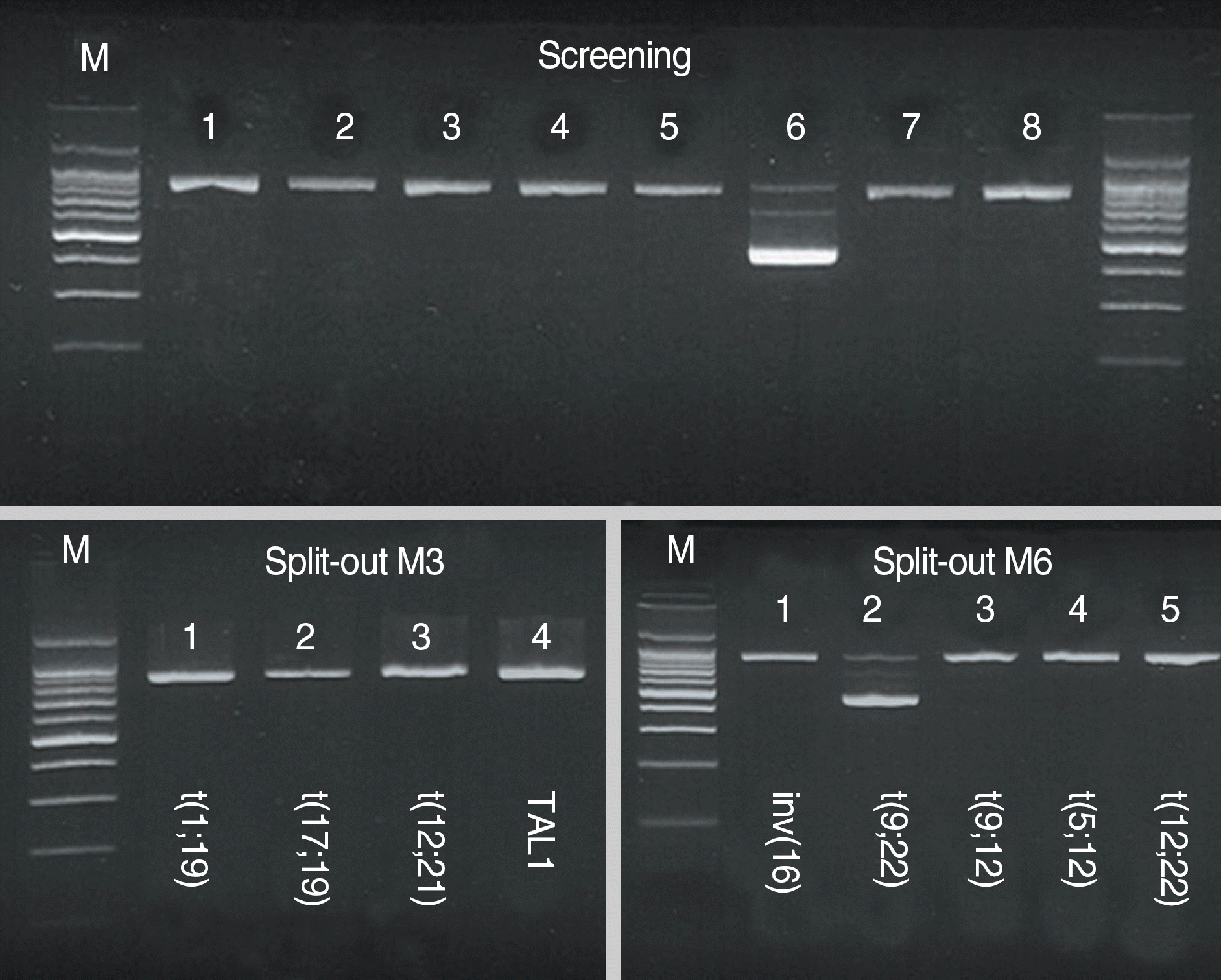 | Fig. 5.Multiplex nested reverse transcriptase PCR using a Hema-Vision kit. The 911-bp bands in each lane represent internal controls. The 472-bp band seen in lane 6 of the screening PCR and lane 2 of the split-out M6 PCR represents the BCR/ABL1 b3a2 transcript. The TCF3/PBX1 transcript is not seen in either the screening or the split-out M3 PCR. |
Table 1.
Clinical and cytogenetic findings of the reported cases of ider(9)(q10)t(9;22)(q34;q11.2)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download