Abstract
Background:
Automated blood cell analyzers often read leukemic blasts as normal cells. In this study, we evaluated the 5-part differential patterns of blasts using automated analyzers to determine if they can differentiate among blast types.
Methods:
Blood samples containing 10% or more blasts were collected from patients with acute leukemia (N=175). The 5-part differential count was conducted using DxH 800 (Beckman Coulter, USA) and XE-2100 analyzers (Sysmex Co., Japan), and the results were compared with manual differential counts, which was used as a reference method.
Results:
The DxH 800 reported the 5-part white blood cell differential count in 98.9% of the cases. The XE-2100 provided an invalid automated differential count in 72% of the cases. Both analyzers counted most lymphoblasts as lymphocytes and most myeloblasts as monocytes. In 11 cases, the DxH 800 reported a 5-part differential count without a blast flag.
Conclusions:
Some automated analyzers are able to recognize and count blasts according to their characteristic cell types. Therefore, complete blood counts obtained automatically can provide valuable data for making provisional decisions regarding the lineage of leukemia cells before further investigation.
Go to : 
REFERENCES
1.McClatchey KD, editor. Clinical laboratory medicine. 2nd ed.Philadelphia: Lippincott Willimas and Wilkins;2002. :809.
2.Briggs C., Longair I., Slavik M., Thwaite K., Mills R., Thavaraja V, et al. Can automated blood film analysis replace the manual differential? An evaluation of the CellaVision DM96 automated image analysis system. Int J Lab Hematol. 2009. 31:48–60.

3.Novis DA., Walsh M., Wilkinson D., St Louis M., Ben-Ezra J. Laboratory productivity and the rate of manual peripheral blood smear review: a College of American Pathologists Q-Probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch Pathol Lab Med. 2006. 130:596–601.

4.Lantis KL., Harris RJ., Davis G., Renner N., Finn WG. Elimination of instrument-driven reflex manual differential leukocyte counts. Optimization of manual blood smear review criteria in a high-volume automated hematology laboratory. Am J Clin Pathol. 2003. 119:656–62.
5.Pierre RV. Peripheral blood film review. The demise of the eyecount leukocyte differential. Clin Lab Med. 2002. 22:279–97.

6.Kakkar N., Kaur R. Utility of white blood cell suspect flags and histogram pattern in the detection of acute leukemia by Advia-60 automated hematology analyzer. Indian J Pathol Microbiol. 2004. 47:322–6.
7.Kline A., Bird A., Adams L., Wale C., Edwards F., Perreira E. Identification of blast cells in the peripheral blood of patients with acute leukaemia using the Technicon H-1. Clin Lab Haematol. 1989. 11:111–6.

8.Hoyer JD., Fisher CP., Soppa VM., Lantis KL., Hanson CA. Detection and classification of acute leukemia by the Coulter STKS Hematology Analyzer. Am J Clin Pathol. 1996. 106:352–8.

9.Swerdlow SH, Campo E, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed.Lyon: IARC;2008. p. 110–78.
10.Gibbs G. Differences in detecting blasts and the role of cytogram analysis. Int J Lab Hematol. 2010. 32:271–2.

11.Inoue H. Overview of automated hematology analyzer XE-2100TM. Sysmex J Int. 1999. 9:58–64.
12.Herklotz R., Huber AR. Precision and Accuracy of the Leukocyte Differential on the Sysmex XE-2100. Sysmex J Int. 2001. 11:8–21.
13.Jean A., Boutet C., Lenormand B., Callat MP., Buchonnet G., Barbay V, et al. The new haematology analyzer DxH800: an evaluation of the analytical performances and leucocyte flags, comparison with the LH755. Int J Lab Hematol. Forthcoming. 2010.
14.Hedley BD., Keeney M., Chin Yee I., Brown W. Initial performance evaluation of the UniCel((R)) DxH 800 Coulter((R)) cellular analysis system. Int J Lab Hematol. Forthcoming. 2010.
Go to : 
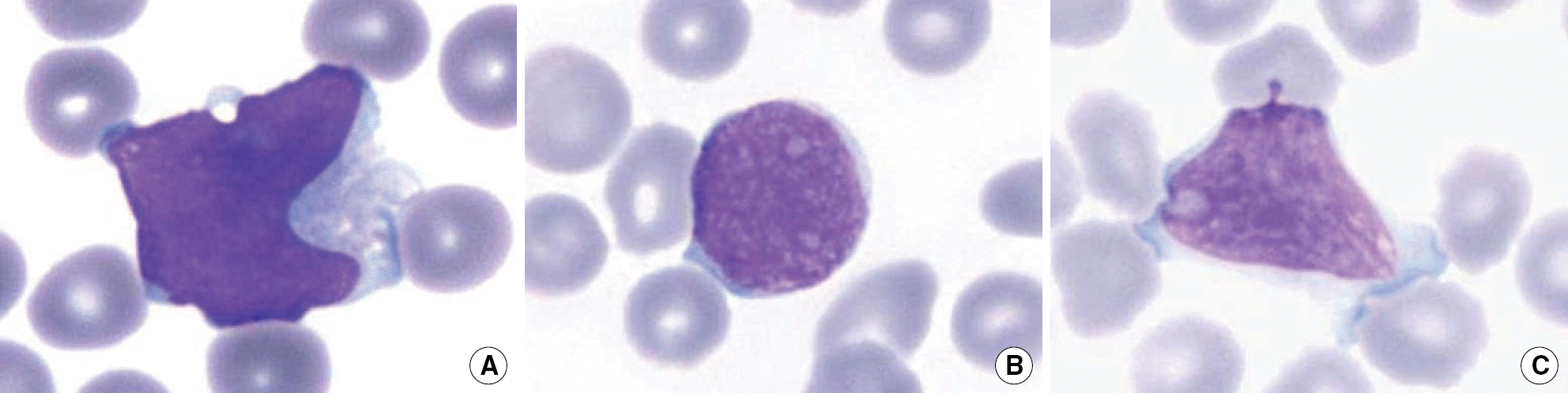 | Fig. 1.(A) A lymphoblast from an ALL case in which the blasts were counted as monocytes in the DxH 800; it showed irregularly shaped nuclei and cytoplasmic vacuoles. (B) A lymphoblast from a case of ALL in which the blasts were counted as lymphocytes in the DxH 800; it showed round nuclei with dispersed chromatin. (C) A lymphoblast from a case of T-ALL that did not generate blast flags; it showed coarse chromatin and abundant cytoplasm. All photomicrographs are ×1,000 magnification. |
Table 1.
Automated differential count results of peripheral blood samples containing 10% or more blasts obtained using the DxH 800 and XE-2100 according to the type of cells which were counted as blasts (% of valid results)
Table 2.
Blast flag results of the peripheral blood samples containing 10% or more blasts obtained using DxH 800 according to the final diagnosis
Table 3.
Total WBC count, percentage of blasts, the cell types that were counted as blasts, and WBC flags in the cases without blast flags by DxH 800




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download