Abstract
Background:
It has been demonstrated that flow cytometric detection of minimal residual disease (MRD) has a prognostic significance in the treatment of patients with acute leukemia. We investigated the significance of flow cytometric MRD detection for the first time in Korea.
Methods:
We analyzed the results of MRD detection in morphologically complete remission bone marrow aspirates from 89 patients with newly-diagnosed or relapsed acute leukemia, in which leukemic cells had cross-lineage antigen expression. Patients were grouped based on MRD frequencies: ≥1.0%, high MRD; <1.0%, low MRD.
Results:
Forty-seven ALL patients consisted of 10 with high and 37 with low MRD levels. Patients with high MRD levels showed a tendency of more frequent relapse than those with low MRD levels (40.0% and 13.5%, respectively) (P=0.08). High MRD group showed a tendency of short relapse-free survival (RFS) and overall survival (OS), although the differences were not statistically significant. Forty-two AML patients consisted of 16 with high and 26 with low MRD levels. There were no correlations between the MRD levels and relapse rate, RFS or OS. AML patients with high MRD levels showed significantly higher rate of unfavorable cytogenetic risk categories and lower rate of favorable risk categories (P=0.03).
Conclusions:
MRD detection by flow cytometric assay of cross-lineage antigen expression would be useful in predicting treatment outcome in patients with ALL rather than AML. We expect that the establishment of the standardization of methods, time to test or antibody combination would be achieved through further trials in this country.
REFERENCES
1.Vidriales MB., San-Miguel JF., Orfao A., Coustan-Smith E., Campana D. Minimal residual disease monitoring by flow cytometry. Best Pract Res Clin Haematol. 2003. 16:599–612.

2.Chung NG., Buxhofer-Ausch V., Radich JP. The detection and significance of minimal residual disease in acute and chronic leukemia. Tissue Antigens. 2006. 68:371–85.

3.Kern W., Haferlach C., Haferlach T., Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Cancer. 2008. 112:4–16.

4.Campana D. Status of minimal residual disease testing in childhood haematological malignancies. Br J Haematol. 2008. 143:481–9.

5.Al-Mawali A., Gillis D., Lewis I. The role of multiparameter flow cytometry for detection of minimal residual disease in acute myeloid leukemia. Am J Clin Pathol. 2009. 131:16–26.

6.Ciudad J., San Miguel JF., Lopez-Berges MC., Vidriales B., Valverde B., Ocqueteau M, et al. Prognostic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol. 1998. 16:3774–81.

7.Coustan-Smith E., Sancho J., Hancock ML., Boyett JM., Behm FG., Raimondi SC, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000. 96:2691–6.

8.Coustan-Smith E., Sancho J., Behm FG., Hancock ML., Razzouk BI., Ribeiro RC, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002. 100:52–8.

9.Dworzak MN., Froschl G., Printz D., Mann G., Potschger U., Muhlegger N, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002. 99:1952–8.

10.Coustan-Smith E., Gajjar A., Hijiya N., Razzouk BI., Ribeiro RC., Rivera GK, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004. 18:499–504.

11.Coustan-Smith E., Ribeiro RC., Stow P., Zhou Y., Pui CH., Rivera GK, et al. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood. 2006. 108:97–102.

12.Borowitz MJ., Devidas M., Hunger SP., Bowman WP., Carroll AJ., Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008. 111:5477–85.

13.San Miguel JF., Martinez A., Macedo A., Vidriales MB., Lopez-Berges C., Gonzalez M, et al. Immunophenotyping investigation of minimal residual disease is a useful approach for predicting relapse in acute myeloid leukemia patients. Blood. 1997. 90:2465–70.

14.Venditti A., Buccisano F., Del Poeta G., Maurillo L., Tamburini A., Cox C, et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. 2000. 96:3948–52.

15.Plata E., Choremi-Papadopoulou H., Viglis V., Yataganas X. Flow-cytometric detection of minimal residual disease with atypical antigen combinations in patients with de novo acute myeloid leukemia. Ann Hematol. 2000. 79:543–6.

16.San Miguel JF., Vidriales MB., Lopez-Berges C., Diaz-Mediavilla J., Gutierrez N., Canizo C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001. 98:1746–51.

17.Venditti A., Tamburini A., Buccisano F., Del Poeta G., Maurillo L., Panetta P, et al. Clinical relevance of minimal residual disease detection in adult acute myeloid leukemia. J Hematother Stem Cell Res. 2002. 11:349–57.

18.Coustan-Smith E., Ribeiro RC., Rubnitz JE., Razzouk BI., Pui CH., Pounds S, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003. 123:243–52.

19.Kern W., Voskova D., Schoch C., Hiddemann W., Schnittger S., Haferlach T. Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood. 2004. 104:3078–85.

20.Kern W., Voskova D., Schoch C., Schnittger S., Hiddemann W., Haferlach T. Prognostic impact of early response to induction therapy as assessed by multiparameter flow cytometry in acute myeloid leukemia. Haematologica. 2004. 89:528–40.
21.Feller N., van der Pol MA., van Stijn A., Weijers GW., Westra AH., Evertse BW, et al. MRD parameters using immunophenotypic detection methods are highly reliable in predicting survival in acute myeloid leukaemia. Leukemia. 2004. 18:1380–90.

22.Buccisano F., Maurillo L., Gattei V., Del Poeta G., Del Principe MI., Cox MC, et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006. 20:1783–9.

23.Shin S., Kahng J., Kim M., Lim J., Kim Y., Han K. Distribution of antigenic aberration in the bone marrow of acute leukemia in complete remission. Korean J Lab Med. 2008. 28:1–7. (신소영, 강지민, 김명신, 임지향, 김용구, 한경자. 급성백혈병 완전 관해 골수에서 항원형 이상 세포의분포. 대한진단검사의학회지 2008;28:1-7.).

24.Kern W., Danhauser-Riedl S., Ratei R., Schnittger S., Schoch C., Kolb HJ, et al. Detection of minimal residual disease in unselected patients with acute myeloid leukemia using multiparameter flow cytometry for definition of leukemia-associated immunophenotypes and determination of their frequencies in normal bone marrow. Haematologica. 2003. 88:646–53.
25.Mrozek K., Heerema NA., Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004. 18:115–36.
Fig. 1.
MRD detection in the bone marrow aspirates from an ALL patient at diagnosis (A), and patients with morphological remission (B and C). The leukemia-associated phenotype includes CD13 and CD19 expression. The MRD levels were 4.33% (B) and 0.01% (C).
Abbreviations: PE, phycoerythrin; FITC, fluorescein isothiocynate; MRD, minimal residual disease.
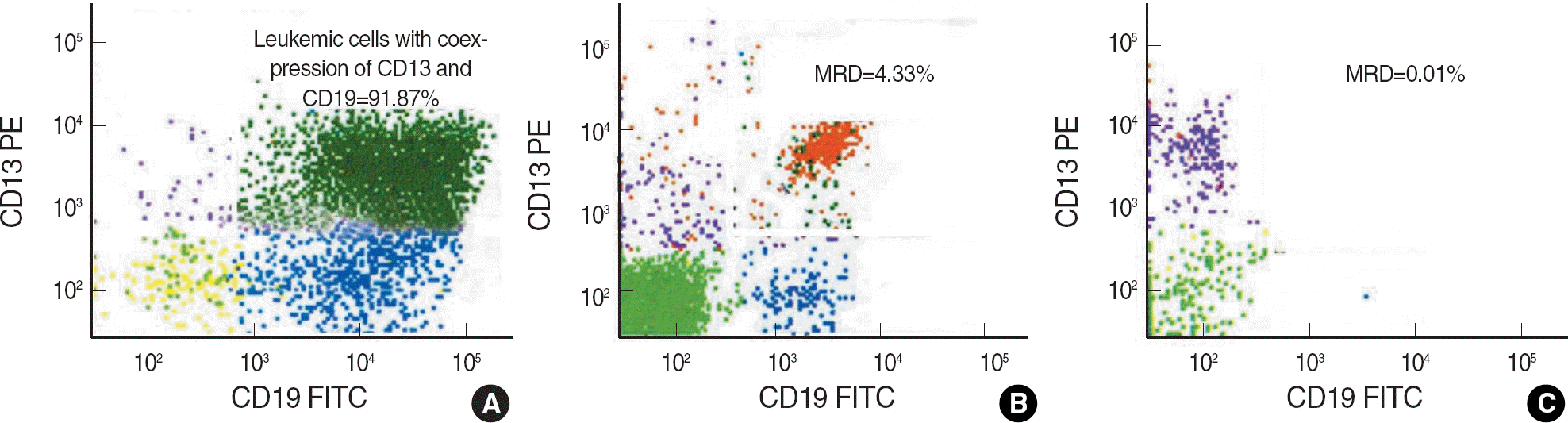
Fig. 2.
Prognostic significance of minimal residual disease (MRD) frequency in bone marrow aspirates after morphological remission in ALL patients. (A) High MRD group showed a tendency of shorter relapse-free survival (RFS) than low MRD group using a cutoff level of 1.0% (P=0.11). (B) High MRD group also showed a tendency of shorter overall survival (OS) than low MRD group (P=0.54).
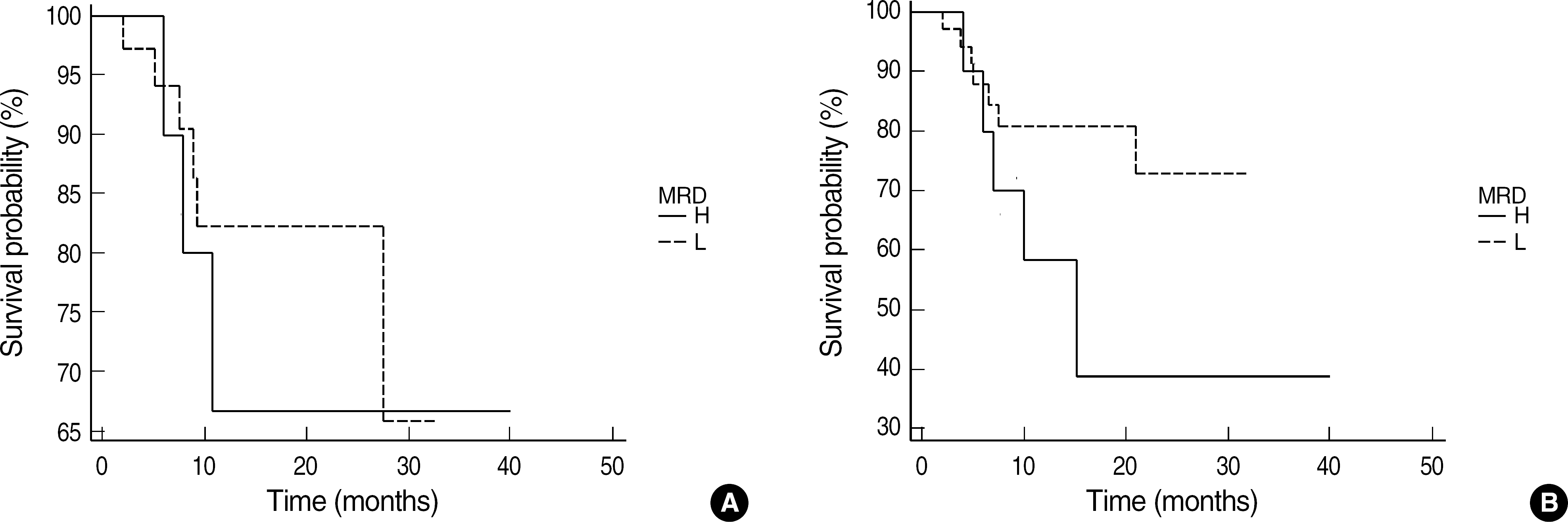
Table 1.
Monoclonal antibody panel for immunophenotyping of acute leukemia
Table 2.
Characteristics of patients with acute leukemia
| Characteristics | ALL patients (N=47) | AML patients (N=42) | ||
|---|---|---|---|---|
| Age (yr) | 7 (1-62) | 41 (1-69) | ||
| Pediatric/adult | 32/15 | 7/35 | ||
| Sex (M/F) | 33/14 | 25/17 | ||
| Cross-lineage antigen expression∗ | CD13 | 20 | CD7 | 14 |
| CD13 & CD33 | 15 | CD56 & CD19 | 8 | |
| CD33 | 5 | CD56 | 8 | |
| Others† | 7 | CD19 | 4 | |
| Others‡ | 8 | |||
| Cytogenetic risk categories | ||||
| Favorable | 19 | 18 | ||
| Intermediate | 12 | 18 | ||
| Unfavorable | 11 | 4 | ||
| Unknown | 5 | 2 | ||
| Follow-up duration (months) | 14 (3-40) | 12 (3-28) |
Table 3.
Clinical characteristics according to the MRD levels
| MRD group | ALL patients | AML patients | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Relapse (%) | RFS (months) | OS (months) | N | Relapse (%) | RFS (months) | OS (months) | |
| High (≥1.0%) | 10 | 4 (40.0)∗ | 9.9 | 10.9 | 16 | 5 (31.3)† | 8.8 | 9.7 |
| Low (<1.0%) | 37 | 5 (13.5)∗ | 13.3 | 13.8 | 26 | 5 (19.2)† | 9.2 | 12.8 |
| High (≥0.5%) | 14 | 4 (28.6) | 10.0 | 10.6 | 22 | 5 (22.7) | 8.4 | 8.8 |
| Low (<0.5%) | 33 | 5 (15.2) | 13.5 | 14.0 | 20 | 5 (25.0) | 9.4 | 13.6 |
| High (≥0.1%) | 35 | 7 (20.0) | 10.0 | 10.5 | 34 | 7 (20.6) | 8.6 | 9.7 |
| Low (<0.1%) | 12 | 2 (16.7) | 16.7 | 16.9 | 8 | 3 (37.5) | 9.6 | 15.5 |
Table 4.
Distribution of the MRD groups according to the time of sample collection
| MRD group | ALL patients | AML patients | ||
|---|---|---|---|---|
| Post-induction (N=26) | Post-consolidation (N=21) | Post-induction (N=27) | Post-consolidation (N=15) | |
| High (≥1.0%) | 3 | 6 | 9 | 7 |
| Low (<1.0%) | 23 | 15 | 18 | 8 |
Table 5.
Relation between MRD groups and cytogenetic risk categories




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download