Abstract
Background:
Carbohydrate-deficient transferrin (CDT) levels have rarely been determined in an Asian population. We evaluated the analytical performance of a test for measuring CDT levels by using capillary electrophoresis (EP).
Methods:
We determined the precision of CDT measurement by using capillary EP and nephelometry and compared the CDT values obtained using both the methods. We included healthy control subjects, abstinent patients with liver disease, and individuals consuming varying amounts of alcohol.
Results:
The CDT measurement by using capillary EP were correlated well with those CDT measurement by using nephelometry, N Latex CDT assay, Y=0.5706X+1.581, R=0.930. The results obtained from both methods showed good qualitative agreement with each other (κ coefficient=0.61). Genetic variants of transferrin isoforms were detected in 4.1% of the tested population. Both the CDT and γ-glutamyl transpeptidase (GGT) levels in the abstinent patients with liver disease were significantly higher than those in healthy abstinent individuals (0.9% vs. 0.5%, 109.5 mg/dL vs. 28.5 mg/dL, respectively), but the difference in CDT values in the 2 groups was less pronounced for the CDT values. Individuals who had a mean daily alcohol intake of more than 60 g/day showed significantly higher CDT levels than those who had a mean daily alcohol intake of less than 60 g/day (1.9% vs. 0.7%, P=0.03).
REFERENCES
1.Stibler H. Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin Chem. 1991. 37:2029–37.

2.Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem. 2001. 47:13–27.

3.Bergstrom JP., Helander A. Clinical characteristics of carbohydrate-deficient transferrin (%disialotransferrin) measured by HPLC: sensitivity, specificity, gender effects, and relationship with other alcohol biomarkers. Alcohol Alcohol. 2008. 43:436–41.

4.Delanghe JR., Helander A., Wielders JP., Pekelharing JM., Roth HJ., Schellenberg F, et al. Development and multicenter evaluation of the N latex CDT direct immunonephelometric assay for serum carbohydrate-deficient transferrin. Clin Chem. 2007. 53:1115–21.

5.Helander A., Husa A., Jeppsson JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003. 49:1881–90.

6.Stibler H., Kjellin KG. Isoelectric focusing and electrophoresis of the CSF proteins in tremor of different origins. J Neurol Sci. 1976. 30:269–85.

7.Helander A., Carlsson S. Carbohydrate-deficient transferrin and gamma-glutamyl transferase levels during disulfiram therapy. Alcohol Clin Exp Res. 1996. 20:1202–5.

8.Jeppsson JO., Kristensson H., Fimiani C. Carbohydrate-deficient transferrin quantified by HPLC to determine heavy consumption of alcohol. Clin Chem. 1993. 39:2115–20.

9.Dibbelt L. Does trisialo-transferrin provide valuable information for the laboratory diagnosis of chronically increased alcohol consumption by determination of carbohydrate-deficient transferrin? Clin Chem. 2000. 46:1203–5.

10.Jeppsson JO., Arndt T., Schellenberg F., Wielders JP., Anton RF., Whitfield JB, et al. Toward standardization of carbohydrate-deficient transferrin (CDT) measurements: I. Analyte definition and proposal of a candidate reference method. Clin Chem Lab Med. 2007. 45:558–62.

11.Schellenberg F., Girre C., Nalpas B., Plat A., Tome A., Pages JC. Analytical evaluation of a new capillary electrophoresis method for carbohydrate-deficient transferrin measurement. Clin Chim Acta. 2007. 382:48–53.

12.Schellenberg F., Mennetrey L., Girre C., Nalpas B., Pages JC. Automated measurement of carbohydrate-deficient transferrin using the Bio-Rad %CDT by the HPLC test on a Variant HPLC system: evaluation and comparison with other routine procedures. Alcohol Alcohol. 2008. 43:569–76.
13.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods: approved guideline EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.
14.Wuyts B., Delanghe JR., Kasvosve I., Wauters A., Neels H., Janssens J. Determination of carbohydrate-deficient transferrin using capillary zone electrophoresis. Clin Chem. 2001. 47:247–55.

15.Arndt T., Hackler R., Kleine TO., Gressner AM. Validation by isoelectric focusing of the anion-exchange isotransferrin fractionation step involved in determination of carbohydrate-deficient transferrin by the CDTect assay. Clin Chem. 1998. 44:27–34.

16.Hackler R., Arndt T., Helwig-Rolig A., Kropf J., Steinmetz A., Schaefer JR. Investigation by isoelectric focusing of the initial carbohydrate-deficient transferrin (CDT) and non-CDT transferrin isoform fractionation step involved in determination of CDT by the ChronAlcoI.D. assay. Clin Chem. 2000. 46:483–92.

17.Lanz C., Kuhn M., Deiss V., Thormann W. Improved capillary electrophoresis method for the determination of carbohydrate-deficient transferrin in patient sera. Electrophoresis. 2004. 25:2309–18.

18.Legros FJ., Nuyens V., Minet E., Emonts P., Boudjeltia KZ., Courbe A, et al. Carbohydrate-deficient transferrin isoforms measured by capillary zone electrophoresis for detection of alcohol abuse. Clin Chem. 2002. 48:2177–86.

19.Fleming MF., Anton RF., Spies CD. A review of genetic, biological, pharmacological, and clinical factors that affect carbohydrate-deficient transferrin levels. Alcohol Clin Exp Res. 2004. 28:1347–55.

20.Scouller K., Conigrave KM., Macaskill P., Irwig L., Whitfield JB. Should we use carbohydrate-deficient transferrin instead of gamma-glutamyltransferase for detecting problem drinkers? A systematic review and metaanalysis. Clin Chem. 2000. 46:1894–902.
21.Imbert-Bismut F., Naveau S., Morra R., Munteanu M., Ratziu V., Abella A, et al. The diagnostic value of combining carbohydrate-deficient transferrin, fibrosis, and steatosis biomarkers for the prediction of excessive alcohol consumption. Eur J Gastroenterol Hepatol. 2009. 21:18–27.

22.Bergstrom JP., Helander A. Influence of alcohol use, ethnicity, age, gender, BMI and smoking on the serum transferrin glycoform pattern: implications for use of carbohydrate-deficient transferrin (CDT) as alcohol biomarker. Clin Chim Acta. 2008. 388:59–67.
23.Lieber CS. Carbohydrate deficient transferrin in alcoholic liver disease: mechanisms and clinical implications. Alcohol. 1999. 19:249–54.
Fig. 1.
Correlation (A) and difference (B) between %CDT levels by capillary electrophoresis and N Latex CDT method. Abbreviation: CDT, carbohydrate-deficient transferring.
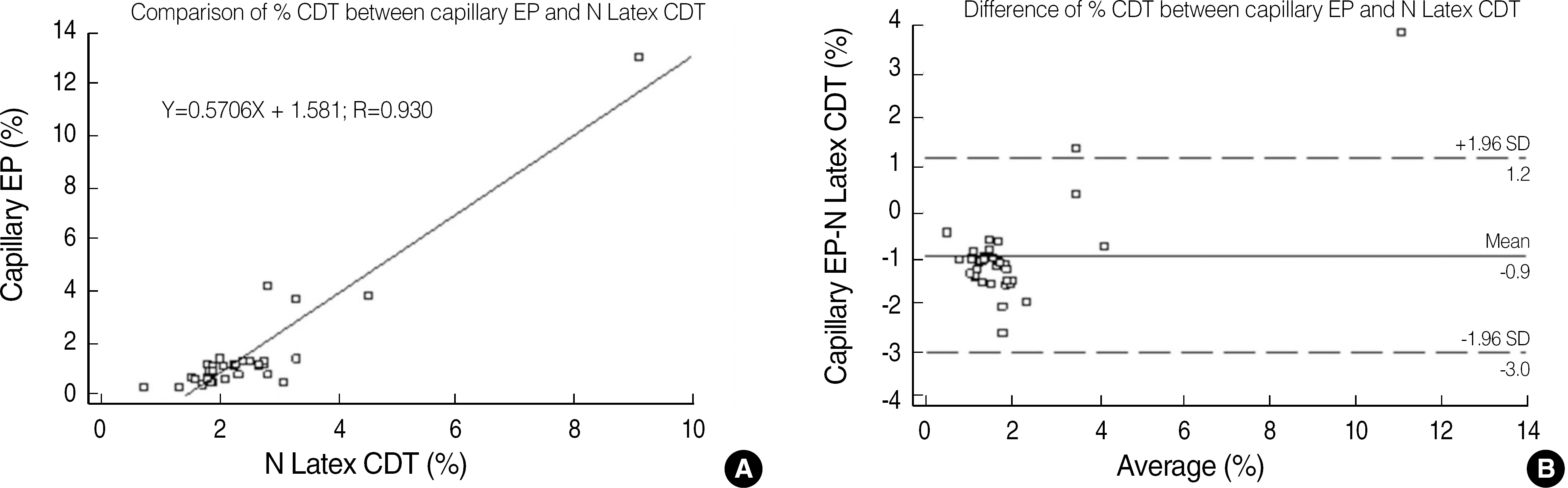
Fig. 2.
Distribution of %CDT using capillary electrophoresis (A) and GGT (B) in healthy abstinent individuals and abstinent patients with liver disease.
Abbreviations: CDT, carbohydrate-deficient transferrin; GGT, γ-glutamyl transpeptidase.
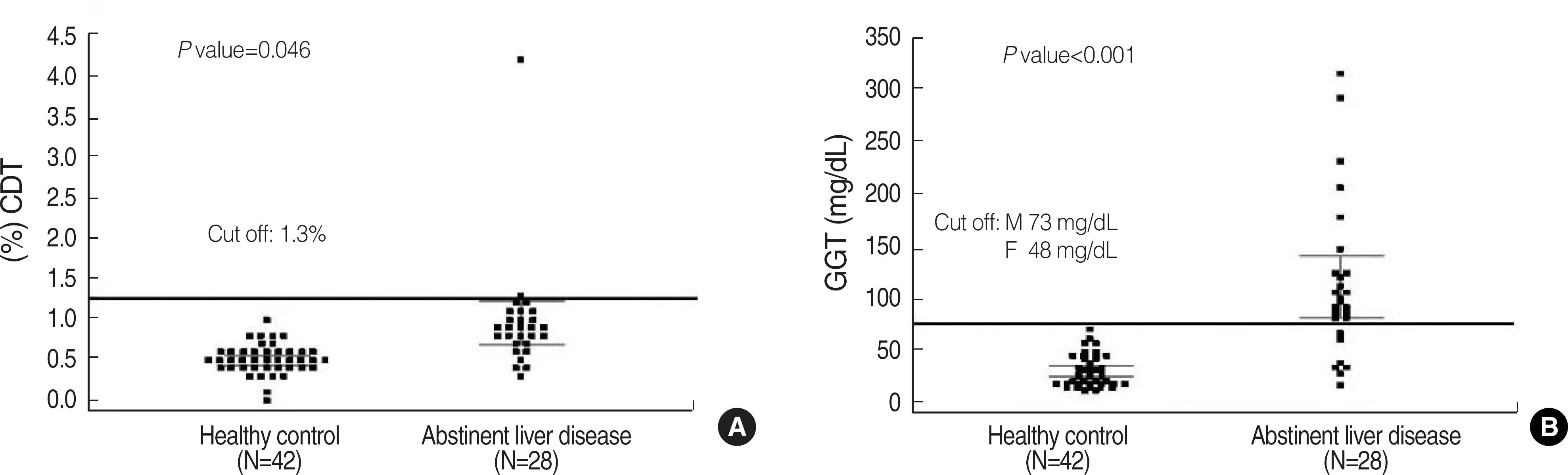
Fig. 3.
Receiver operating characteristic curves of %CDT using capillary electrophoresis for detection of alcohol consumptions according to each target amount of daily alcohol consumption (A: mean alcohol 40 g/day or more, B: 60 g/day or more, C: 120 g/day or more, N=83).
Abbreviation: AUC, area under ROC curves.
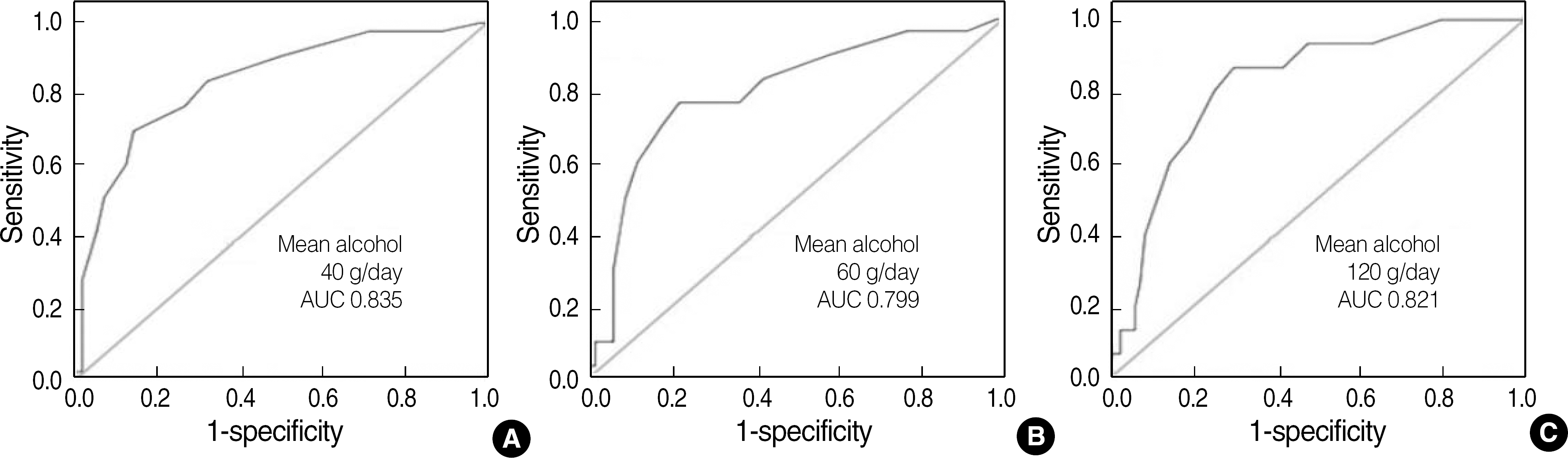
Table 1.
Precision of %CDT using capillary electrophoresis
| Analyte | Mean (%) | Within-run precision SD (CV, %) | Total precision SD (CV, %) |
|---|---|---|---|
| Normal control | 0.51 | 0.04 (6.9) | 0.03 (6.6) |
| Abnormal control | 6.96 | 0.13 (1.8) | 0.31 (4.5) |
Table 2.
Distribution of %CDT using capillary electrophoresis and GGT in healthy abstinent individuals and abstinent patients with liver disease
Table 3.
Distribution of %CDT values according to mean alcohol consumption
Table 4.
Distribution of %CDT values according to number of abstinent days
| Abstinent day | N of patients | %CDT | P value | |
|---|---|---|---|---|
| Mean (SD) | Median (range) | |||
| ≤3d | 20 | 2.0 (2.7) | 1.1 (0.5-13.0) | 0.110∗ |
| ≥7d | 12 | 1.0(0.4) | 1.0(0.3-1.4) | 0.314† |
| >7d | 12 | 0.9 (0.3) | 1.1 (0.5-1.3) | |
Table 5.
Sensitivities and specificities of %CDT using capillary electrophoresis at different cut-off limits for detecting each amount of mean alcohol consumptions (N=83)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download