Abstract
Background:
Since April 2009, novel influenza A (H1N1) infection is spreading throughout the world. This infection might be fatal for immunocompromised patients who are at a potentially high risk of developing infectious complications. We investigated the detection rate and features of H1N1 infection in immunocompromised patients.
Methods:
Between August 2009 and February 2010, we examined 8,112 subjects, including 390 immunocompromised patients, for H1N1. Swab samples were taken from the nose and throat of the participants. Real-time PCR was performed to identify H1N1 viral genes.
Results:
Positive results were obtained in 2,953/8,112 (36.4%) subjects and 46/390 (11.8%) immuno-compromised patients. H1N1 was identified in 8.7% patients with solid cancer, 12.9% patients with hematologic malignancy, 16.7% patients with chronic renal disease, and 14.5% patients with kidney transplantation. The mean cycle threshold (Ct) value of PCR was significantly lower (P<0.05) in patients with hematologic malignancy as compared to that in patients with chronic renal disease and control subjects. Four patients died due to respiratory complications.
Go to : 
REFERENCES
1.Centers for Disease Control and Prevention. Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:467–70.
2.Chan M. World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en. (Updated on Jun. 2009.
3.Jain S., Kamimoto L., Bramley AM., Schmitz AM., Benoit SR., Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009. 361:1935–44.

4.Dawood FS., Jain S., Finelli L., Shaw MW., Lindstrom S., Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009. 360:2605–15.

5.Centers for Disease Control and Prevention. Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection - United States, April-August 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:941–7.
6.Kunisaki KM., Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009. 9:493–504.

7.Lapinsky SE. H1N1 novel influenza A in pregnant and immuno-compromised patients. Crit Care Med. 2010. 38:e52–7.

8.Couch RB., Englund JA., Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997. 102:2–9.
9.Clements ML., Betts RF., Tierney EL., Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986. 24:157–60.

10.Gooskens J., Jonges M., Claas EC., Meijer A., Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis. 2009. 199:1435–41.

11.Chemaly RF., Ghosh S., Bodey GP., Rohatgi N., Safdar A., Keating MJ, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore). 2006. 85:278–87.
12.Nichols WG., Guthrie KA., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004. 39:1300–6.

13.Redelman-Sidi G., Sepkowitz KA., Huang CK., Park S., Stiles J., Eagan J, et al. 2009 H1N1 influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J Infect. 2010. 60:257–63.

14.Seiter K., Nadelman RB., Liu D., Ahmed T., Montecalvo MA. Novel influenza A (H1N1) in patients with hematologic malignancies. J Clin Oncol. 2010. 28:e27–9.

15.Kharfan-Dabaja MA., Velez A., Richards K., Greene JN., Field T., Sandin R. Influenza A/pandemic 2009/H1N1 in the setting of allogeneic hematopoietic cell transplantation: a potentially catastrophic problem in a vulnerable population. Int J Hematol. 2010. 91:124–7.

16.Watcharananan SP., Suwatanapongched T., Wacharawanichkul P., Chantratitaya W., Mavichak V., Mossad SB. Influenza A/H1N1 2009 pneumonia in kidney transplant recipients: characteristics and outcomes following high-dose oseltamivir exposure. Transpl Infect Dis. 2010. 12:127–31.

17.Kumar A., Zarychanski R., Pinto R., Cook DJ., Marshall J., Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009. 302:1872–9.

Go to : 
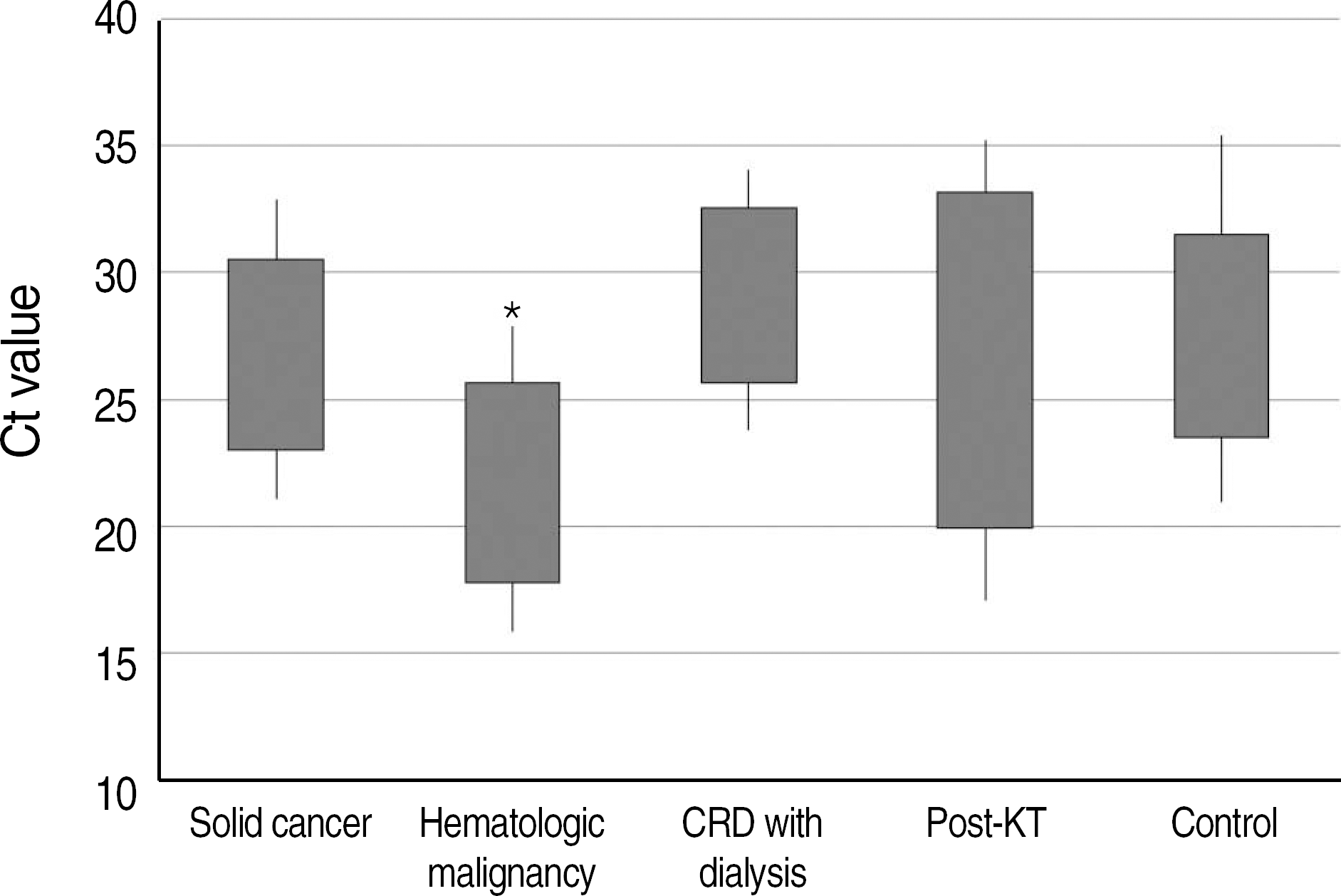 | Fig. 1.Fig. 1. Comparison of cycle threshold (Ct) values of real-time PCR. ∗Significantly lower than CRD with dialysis and control (P<0.05). Abbreviations: CRD, chronic renal disease; KT, kidney transplantation. |
Table 1.
Detection rate of novel influenza A (H1N1) virus in suspected patients
| N of patients examined | N of patients with positive result (%) | |
|---|---|---|
| Total patients | 8,112 | 2,953 (36.4) |
| Immunocompromised | 390 | 46 (11.8)∗ |
| Solid cancer | 195 | 17 (8.7) |
| Hematologic malignancy | 62 | 8 (12.9) |
| CRD with dialysis | 78 | 13 (16.7) |
| Post-KT | 55 | 8 (14.5) |
Table 2.
Characteristics of 46 immunocompromised patients infected with novel influenza A (H1N1) virus
Table 3.
Characteristics of deceased patients with novel influenza A (H1N1) infection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download