Abstract
Background:
Thyroid cancer patients should be on low-iodine diet (LID) before radioactive iodine therapy (RAIT) to maximize the effect of RAIT. Urinary iodine excretion is the most accurate marker of very recent dietary iodine intake. We developed and evaluated the analytical performance of inductively coupled plasma-mass spectrometry (ICP-MS) to determine urinary iodine concentration.
Methods:
We evaluated the linearity, precision, accuracy, and lower limit of quantification (LLOQ) of an ICP-MS method (Agilent 7500ce) to determine urinary iodine concentration in accordance with the Food and Drug Administration (FDA) guidelines for bioanalytical method validation. This method was used to determine and compare the iodine concentration in random urine samples of 120 thyroid cancer patients on LID for 1 week and 80 healthy adults on normal diet.
Results:
Our ICP-MS method showed good linearity (1.0-1,913 mg/L; R2>0.999). Both intra-day and inter-day precision CV were within 20% for the LLOQ (1 mg/L) and within 15% for the other concentrations. Accuracy was 110-120% for the LLOQ and 95-115% for the other concentrations. The median concentration of iodine in random urine samples from thyroid cancer patients on LID (38.7 mg/L) was significantly lower than that of healthy subjects (238.8 mg/L) (P<0.0001).
Conclusions:
Urinary iodine analysis by ICP-MS showed good linearity, precision, accuracy, wide measuring range of detection, and lower LLOQ. This method will be very useful to evaluate the status of dietary iodine intake and the appropriateness of LID in thyroid cancer patients, thereby maximizing the effect of RAIT.
REFERENCES
1.Cooper DS., Doherty GM., Haugen BR., Kloos RT., Lee SL., Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006. 16:109–42.

2.Luster M., Clarke SE., Dietlein M., Lassmann M., Lind P., Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008. 35:1941–59.

3.Sawka AM., Thephamongkhol K., Brouwers M., Thabane L., Browman G., Gerstein HC. Clinical review 170: a systematic review and meta-analysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004. 89:3668–76.
4.Cavalieri RR. Iodine metabolism and thyroid physiology: current concepts. Thyroid. 1997. 7:177–81.

5.Andersson M., Takkouche B., Egli I., Allen HE., de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005. 83:518–25.
6.Zimmermann MB. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. J Trace Elem Med Biol. 2008. 22:81–92.

7.Demers LM., Spencer CA. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf). 2003. 58:138–40.

8.Caldwell KL., Maxwell CB., Makhmudov A., Pino S., Braverman LE., Jones RL, et al. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem. 2003. 49:1019–21.

9.Macours P., Aubry JC., Hauquier B., Boeynaems JM., Goldman S., Moreno-Reyes R. Determination of urinary iodine by inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. 2008. 22:162–5.

10.May SL., May WA., Bourdoux PP., Pino S., Sullivan KM., Maberly GF. Validation of a simple, manual urinary iodine method for estimating the prevalence of iodine-deficiency disorders, and interlaboratory comparison with other methods. Am J Clin Nutr. 1997. 65:1441–5.

11.U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: bioanalytical method validation. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration;2001.
12.Guan H., Ji M., Bao R., Yu H., Wang Y., Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009. 94:1612–7.

13.Teng W., Shan Z., Teng X., Guan H., Li Y., Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006. 354:2783–93.

14.Harach HR., Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 2008. 19:209–20.

15.Kim JY., Kim KR. Dietary iodine intake and urinary iodine excretion in patients with thyroid diseases. Yonsei Med J. 2000. 41:22–8.

16.Pennington JA., Young BE. Total diet study nutritional elements, 1982-1989. J Am Diet Assoc. 1991. 91:179–83.

17.Lee SM., Lewis J., Buss DH., Holcombe GD., Lawrance PR. Iodine in British foods and diets. Br J Nutr. 1994. 72:435–46.

18.Laurberg P., Pedersen KM., Hreidarsson A., Sigfusson N., Iversen E., Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998. 83:765–9.

20.Ristic-Medic D., Piskackova Z., Hooper L., Ruprich J., Casgrain A., Ashton K, et al. Methods of assessment of iodine status in humans: a systematic review. Am J Clin Nutr. 2009. 89:2052S–69S.

21.Rasmussen LB., Ovesen L., Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999. 53:401–7.

22.Als C., Helbling A., Peter K., Haldimann M., Zimmerli B., Gerber H. Urinary iodine concentration follows a circadian rhythm: a study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab. 2000. 85:1367–9.

23.Andersen S., Karmisholt J., Pedersen KM., Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008. 99:813–8.

24.Knudsen N., Christiansen E., Brandt-Christensen M., Nygaard B., Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur J Clin Nutr. 2000. 54:361–3.
25.WHO; UNICEF; ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed.Geneva: WHO;2007.
26.Caldwell KL., Jones R., Hollowell JG. Urinary iodine concentration: United States National Health And Nutrition Examination Survey 2001-2002. Thyroid. 2005. 15:692–9.

27.Lee SY., Oh HJ., Choi YH., Kim JW., Kim SH. Trace metal analysis using inductively coupled plasma-mass spectrometry (ICP-MS). Korean J Lab Med. 2004. 24:362–70. (이수연, 오현주, 최윤호, 김종원,김선희.유도결합플라즈마질량분석기를 이용한 체내 미량금속 분석.대한진단검사의학회지 2004;24:362-70.).
28.Hsiung CS., Andrade JD., Costa R., Ash KO. Minimizing interferences in the quantitative multielement analysis of trace elements in biological fluids by inductively coupled plasma mass spectrometry. Clin Chem. 1997. 43:2303–11.

29.Haldimann M., Zimmerli B., Als C., Gerber H. Direct determination of urinary iodine by inductively coupled plasma mass spectrometry using isotope dilution with iodine-129. Clin Chem. 1998. 44:817–24.

30.Pabla D., Akhlaghi F., Ahmed A., Zia H. Development and validation of an inductively coupled plasma mass spectrometry method for quantification of levothyroxine in dissolution studies. Rapid Commun Mass Spectrom. 2008. 22:993–6.

Fig. 1.
Linearity of standard iodine obtained by using inductively coupled plasma-mass spectrometry (ICP-MS).
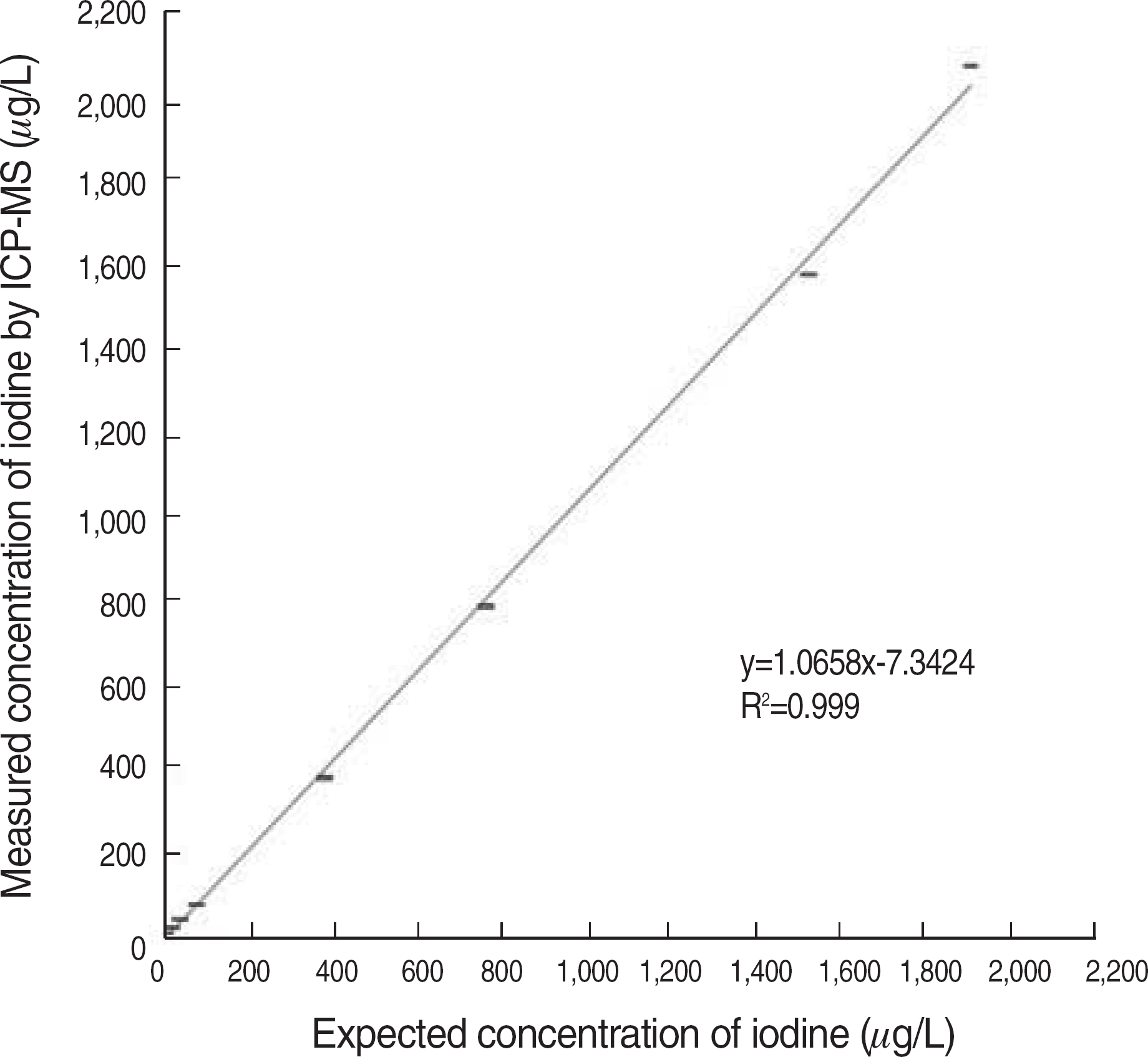
Fig. 2.
Distribution patterns of urinary iodine in normal adults and thyroid cancer patients on low-iodine diet. Abbreviation: ICP-MS, inductively coupled plasma-mass spectrometry.
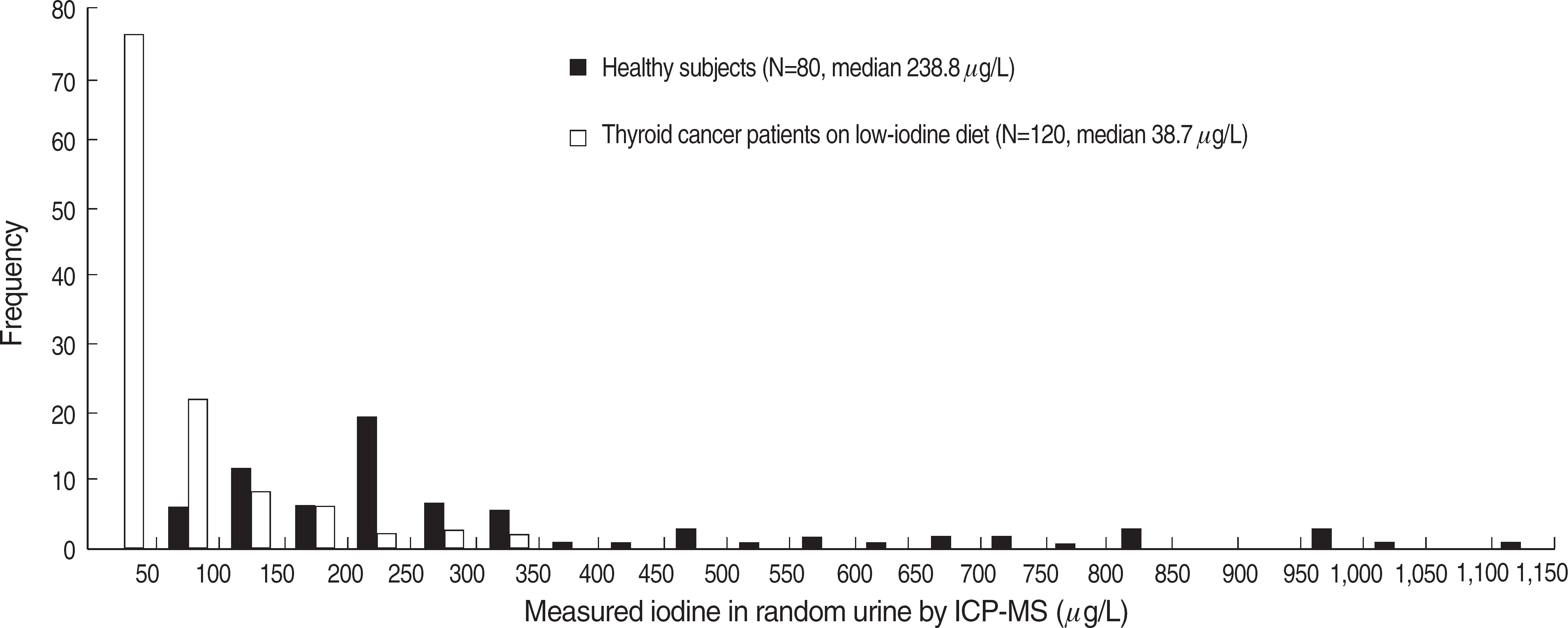
Table 1.
Instrumental parameters and measurement conditions
Table 2.
Precision and accuracy data for standard iodine obtained by using inductively coupled plasma-mass spectrometry




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download