Abstract
Background:
The purpose of this study was to evaluate the performance and agreement among HbA1c values measured using selected analyzers certified by the National Glycohemoglobin Standardization Program (NGSP) and standardized by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
Methods:
HbA1c determined using D-10 (Bio-Rad, USA), Variant II Turbo (Turbo; Bio-Rad, USA), Cobas Integra 800 (Integra; Roche, Switzerland) and Afinion AS100 (Afinion; Axis-Shield, Norway) were compared with each other. Precision and method comparisons with Deming regression were evaluated according to CLSI recommendations. We also compared the HbA1c values obtained with each analyzer using either IFCC or NGSP methods by correlation analysis and kappa statistics.
Results:
The repeatability and method/device precisions of D-10 and Afinion were acceptable. The correlation coefficients of HbA1c were 0.986 for D-10 vs. Afinion, 0.997 for D-10 vs. Turbo, 0.988 for D-10 vs. Integra, and 0.991 for Integra vs. Afinion. The average biases of HbA1c Afinion (IFCC) and HbA1c Integra (IFCC) against HbA1c D-10 (NGSP) were −1.90% and −1.79%, respectively. Kappa agreement statistics for the three diabetic control group HbA1c values of “less than 6.5%,” “6.5%-7.5%,” and “greater than 7.5%” for D-10 vs. Turbo, D-10 vs. Integra, and D-10 vs. Afinion were 0.872, 0.836, and 0.833, respectively.
Go to : 
REFERENCES
1.Nathan DM., Singer DE., Hurxthal K., Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984. 310:341–6.

2.Benjamin RJ., Sacks DB. Glycated protein update: implications of recent studies, including the diabetes control and complications trial. Clin Chem. 1994. 40:683–7.

3.Steffes M., Cleary P., Goldstein D., Little R., Wiedmeyer HM., Rohlfing C, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005. 51:753–8.

4.Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE; NGSP Steering Committee. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001. 47:1985–92.
5.Jeppsson JO., Kobold U., Barr J., Finke A., Hoelzel W., Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002. 40:78–89.

6.Kobold U., Jeppsson JO., Dülffer T., Finke A., Hoelzel W., Miedema K. Candidate reference methods for hemoglobin A1c based on peptide mapping. Clin Chem. 1997. 43:1944–51.

7.Hoelzel W., Weykamp C., Jeppsson JO., Miedema K., Barr JR., Goodall I, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004. 50:166–74.

9.Penttilä IM., Halonen T., Punnonen K., Tiikkainen U. Best use of the recommended IFCC reference method, material and values in HbA1C analyses. Scand J Clin Lab Invest. 2005. 65:453–62.
10.Mosca A., Goodall I., Hoshino T., Jeppsson JO., John WG., Little RR, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med. 2007. 45:1077–80.

11.Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1c measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007. 30:2399–400.
12.Marzullo C., Minery M. Evaluation of D10 hemoglobin testing system for hemoglobin A1c assay. Ann Biol Clin (Paris). 2008. 66:95–9.
13.Leon-Justel A., Santotoribio JD., Dominguez-Pascual I., Delgado AL., Macias C., Herrera MT, et al. Influence of reduction in the elution times on HPLC glycohaemoglobin results. Clin Biochem. 2009. 42:1582–4.

14.Fleming JK. Evaluation of HbA1c on the Roche COBAS Integra 800 closed tube system. Clin Biochem. 2007. 40:822–7.

15.Tholen DW, Kallner A, editors. Evaluation precision performance of quantitative measurement method: approved guideline. CLSI document EP5-A2. 2nd ed.Wayne, PA: NCCLS;2004.
16.Krouwer JS, Tholen DW, editors. Method comparison and bias estimation using patient samples: approved guideline. CLSI document EP9-A2. 2nd ed.Wayne, PA: NCCLS;2002.
17.Song JH., Kwon KC., Kim JH., Kim JW., Min WK., Lee SY, et al. Annual report on external quality assessment in metabolic disorders in Korea (2008). J Lab Med Qual Assur. 2009. 31:143–59. (송정한, 권계철,
김정호, 김종원, 민원기, 이수연 등. 대사질환검사 신빙도조사 결과보고(2008). 임상검사와정도관리 2009;31:143-59.).
18.Goldstein DE, Chenault VM, editors. Harmonization of gylcohemoglobin measurement. CLSI document C44-A. Wayne, PA: NCCLS;2002.
19.Miedema K. Standardization of HbA1c and optimal range of monitoring. Scand J Clin Lab Invest Suppl. 2005. 240:61–72.

20.Dhatt GS., Agarwal MM., Bishawi B. HbA1c: a comparison of NGSP with IFCC transformed values. Clin Chim Acta. 2005. 358:81–6.

Go to : 
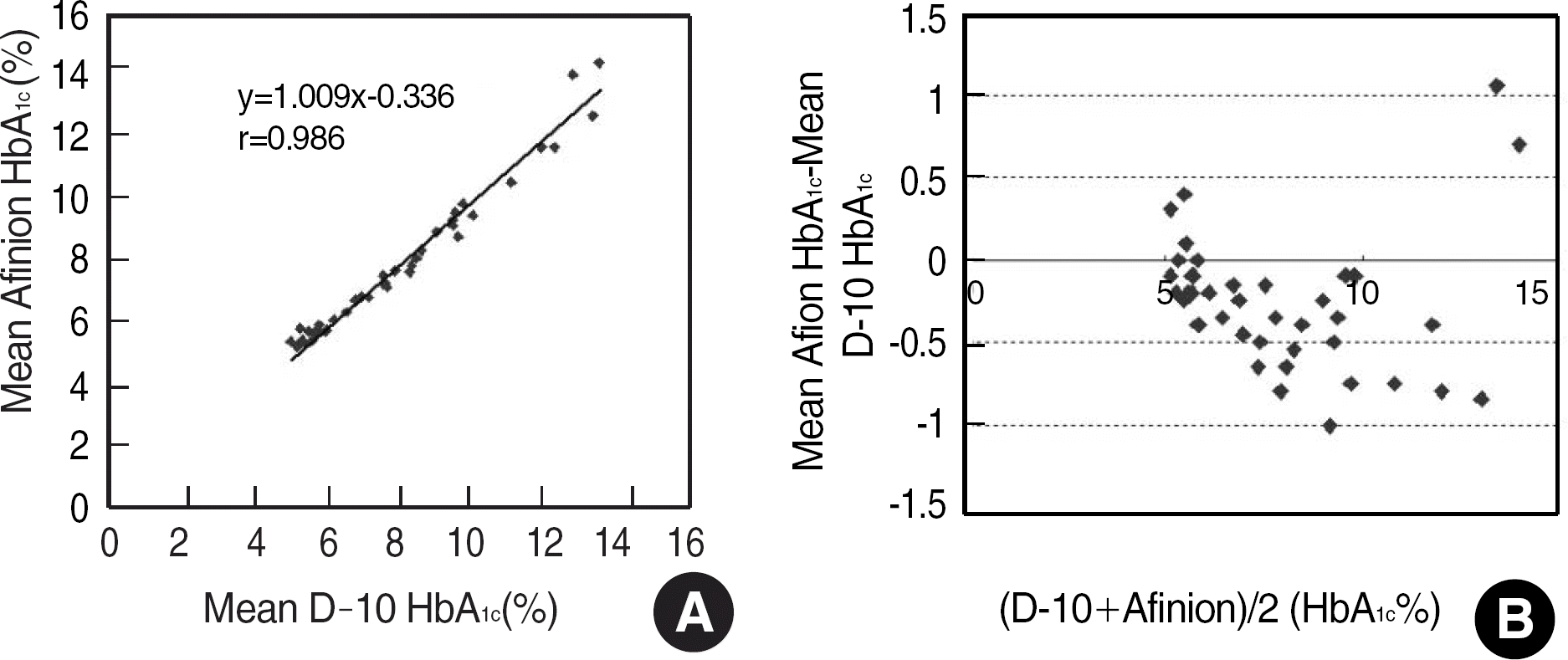 | Fig. 1.Comparison of the HbA1c method (A) using Afinion AS100 and D-10, which showed good correlation, but (B) with slight negative bias in Afinion AS100 HbA1c against D-10 evaluated according to CLSI EP9-A2 guidelines. |
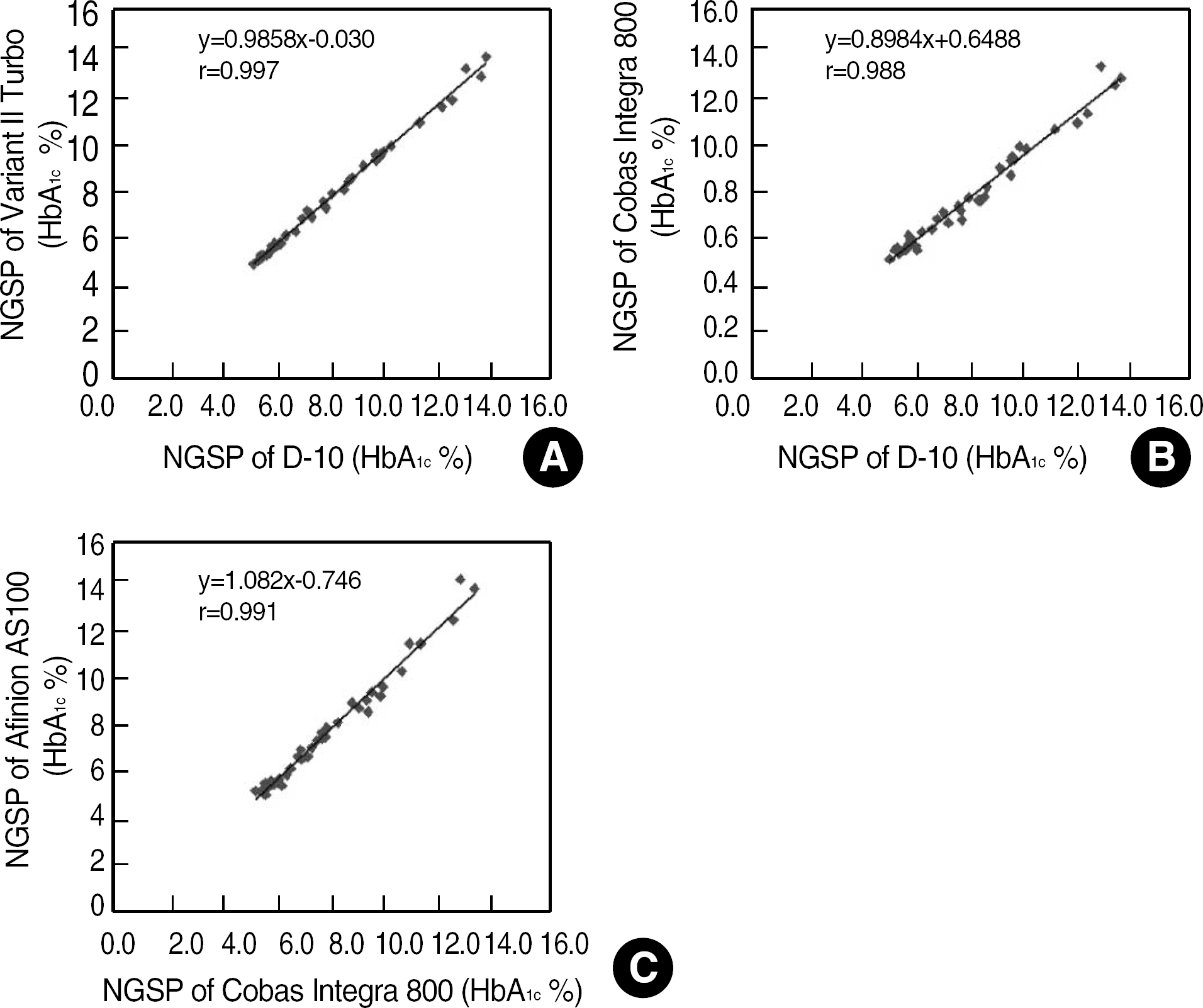 | Fig. 2.Comparison of HbA1c values using each NGSP method. (A) Variant II Turbo vs. D-10, (B) Cobas Integra 800 vs. D-10, and (C) Afinion AS100 vs. Cobas Integra 800, which all showed good correlation.
Abbreviation: NGSP, National Glycohemoglobin Standardization Program.
|
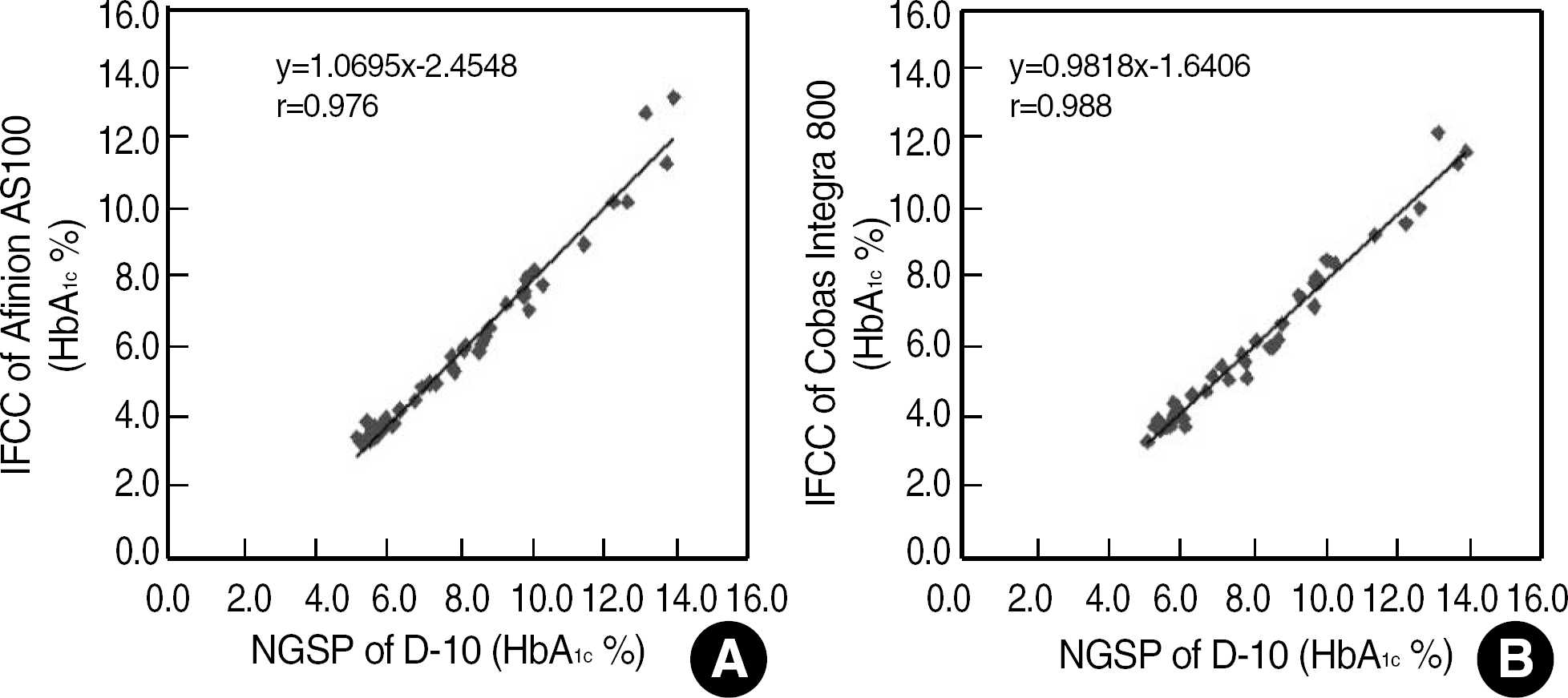 | Fig. 3.Comparison of HbA1c methods. (A) Afinion AS100 (IFCC) vs. D-10 (NGSP) and (B) Cobas Integra 800 (IFCC) vs. D-10 (NGSP), which show large negative differences (−1.90% and − 1.79%, respectively).
Abbreviations: NGSP, National Glycohemoglobin Standardization Program; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine.
|
Table 1.
Characteristics of D-10, Variant II Turbo, Cobas Integra 800, and Afinion AS100 HbA 1c analyzers
Table 2.
Repeatability, device/method precision, and between-day precision of D-10 and Afinion AS100 HbA1c analyzers evaluated by CLSI EP5-A2




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download