Abstract
Background:
Bisalbuminemia is a hereditary or an acquired condition characterized by the presence of 2 albumin variants with different mobilities on serum protein electrophoresis (SPE). The clinical significance of bisalbuminemia has not been clearly established. However, some regions of the albumin variant may affect the biochemical analysis of biomolecules such as steroid or thyroid hormones by altering their albumin-binding affinities. In this study, we analyzed the clinical manifestations, genetic variations, and the albumin-binding characteristics in Korean patients with bisalbuminemia.
Methods:
We performed SPE for samples from 580 Korean subjects and identified bisalbuminemia on the basis of the results of SPE. The clinical and biochemical characteristics, ALB gene mutations, and the structures of the albumin variants of patients with bisalbuminemia were analyzed.
Results:
SPE showed bisalbuminemia in 2 patients. One patient showed a genetic variation known as Nagasaki-1 (Asp293Gly) and the other showed a hitherto unreported missense mutation (c.593A>T; Lys198Ile). In both cases, the serum concentrations of the substances with binding affinity for albumin were not affected, and the mutation sites of the albumin were not located with the protein-binding loci.
Conclusions:
The 2 Korean patients with bisalbuminemia showed genetic variations, including a novel missense mutation. The ALB gene analysis with 3D modeling is useful for determining the nature of bisalbuminemia and for predicting the effects on the albumin-binding affinity of other biochemical compounds.
Go to : 
REFERENCES
1.Hoang MP., Baskin LB., Wians FH Jr. Bisalbuminuria in an adult with bisalbuminemia and nephrotic syndrome. Clin Chim Acta. 1999. 284:101–7.

2.Le Treut A., Catheline M., Cloarec L. Inherited and familial bisalbuminemia. Acquired transient bisalbuminemia. Pathol Biol (Paris). 1977. 25:45–55.
3.Dubost JJ., Deseubis T., Sauvezie B., Gentou C., Rampon S. A tricuspid electrophoretic pattern: familial bisalbuminemia with monoclonal immunoglobulin. Pathol Biol (Paris). 1985. 33:837–8.
4.Arai K., Ishioka N., Huss K., Madison J., Putnam FW. Identical structural changes in inherited albumin variants from different populations. Proc Natl Acad Sci U S A. 1989. 86:434–8.

5.Giuliani A., Hassan HJ., Casalbore P., Marini L., Orlando M., Tentori L. Structural or functional heterogeneity of normal human serum albumin, allo albumin, bisalbumin. Clin Chim Acta. 1981. 113:43–9.

6.Huss K., Putnam FW., Takahashi N., Takahashi Y., Weaver GA., Peters T Jr. Albumin Cooperstown: a serum albumin variant with the same (313 Lys——Asn) mutation found in albumins in Italy and New Zealand. Clin Chem. 1988. 34:183–7.

7.Carlson J., Sakamoto Y., Laurell CB., Madison J., Watkins S., Putnam FW. Alloalbuminemia in Sweden: structural study and phenotypic distribution of nine albumin variants. Proc Natl Acad Sci U S A. 1992. 89:8225–9.

8.Dyan S., Damasio M., El Ouaer L., Lurie A. Bisalbuminemia in pancreatitis without serous effusion or pseudocyst. Nouv Presse Med. 1981. 10:1746.
9.Arvan DA., Blumberg BS., Melartin L. Transient “bisalmuminemia” induced by drugs. Clin Chim Acta. 1968. 22:211–8.

10.Kragh-Hansen U., Minchiotti L., Brennan SO., Sugita O. Hormone binding to natural mutants of human serum albumin. Eur J Biochem. 1990. 193:169–74.

11.Kragh-Hansen U., Brennan SO., Galliano M., Sugita O. Binding of warfarin, salicylate, and diazepam to genetic variants of human serum albumin with known mutations. Mol Pharmacol. 1990. 37:238–42.
12.Arai K., Madison J., Huss K., Ishioka N., Satoh C., Fujita M, et al. Point substitutions in Japanese alloalbumins. Proc Natl Acad Sci U S A. 1989. 86:6092–6.

13.Brennan SO., Herbert P. Albumin Canterbury (313 Lys——Asn). A point mutation in the second domain of serum albumin. Biochim Biophys Acta. 1987. 912:191–7.
14.Brennan SO. The molecular abnormality of albumin Parklands: 365 Asp——His. Biochim Biophys Acta. 1985. 830:320–4.
15.Sunthornthepvarakul T., Angkeow P., Weiss RE., Hayashi Y., Refetoff S. An identical missense mutation in the albumin gene results in familial dysalbuminemic hyperthyroxinemia in 8 unrelated families. Biochem Biophys Res Commun. 1994. 202:781–7.
16.Petersen CE., Scottolini AG., Cody LR., Mandel M., Reimer N., Bhagavan NV. A point mutation in the human serum albumin gene results in familial dysalbuminaemic hyperthyroxinaemia. J Med Genet. 1994. 31:355–9.

17.Madison J., Arai K., Sakamoto Y., Feld RD., Kyle RA., Watkins S, et al. Genetic variants of serum albumin in Americans and Japanese. Proc Natl Acad Sci U S A. 1991. 88:9853–7.

Go to : 
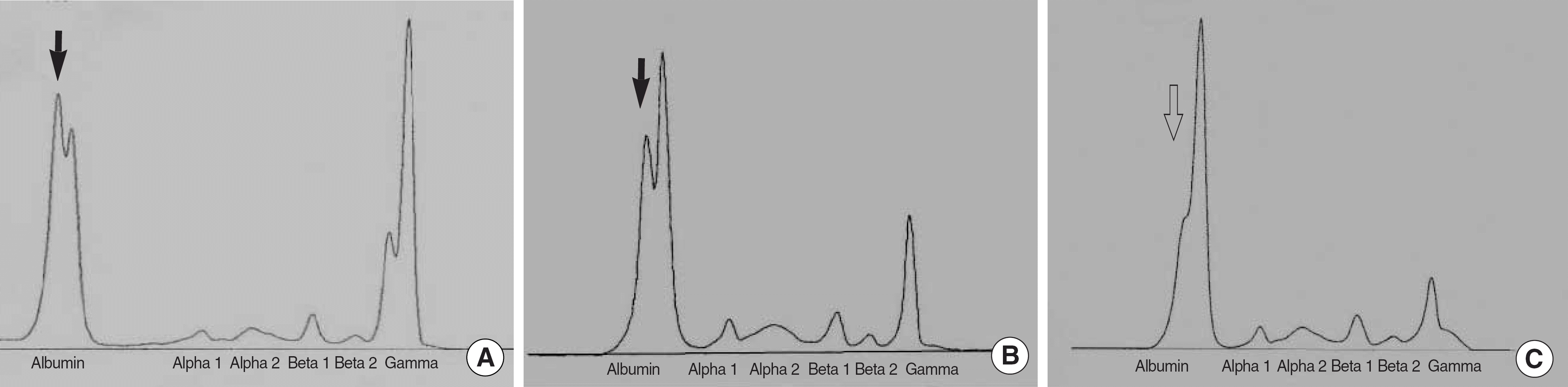 | Fig. 1.Capillary electrophoreses of the serum proteins of patient 1 (A) and patient 2 (B) with bisalbuminemia. Two peaks were observed at the albumin region. Solid arrows indicate the characteristic peak of albumin. A 1:1 mixture of the serum of normal control and that of patient 2 showed an abnormal albumin peak (open arrow) with a low peak height (C). |
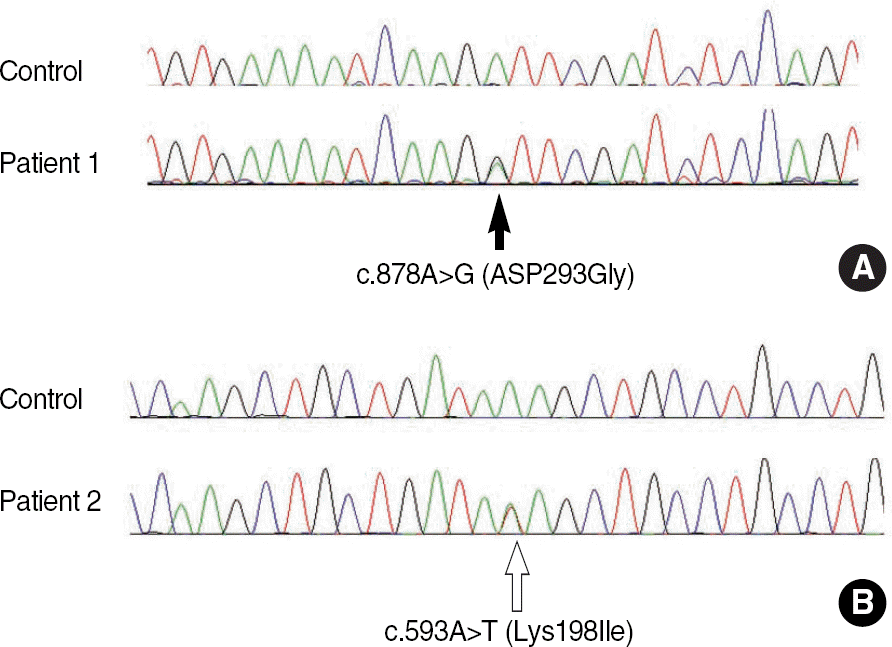 | Fig. 2.Mutation analysis of the albumin (ALB) gene. Direct sequencing of the ALB gene shows overlapping peaks (arrow) at nucleotide position 878 in patient 1 due to a heterozygous A to G transition (c.878A>G; D298G) (A) and at the nucleotide position 593 in the patient 2 due to a heterozygous A to T transversion (c.593A>T; K198I) (B). |
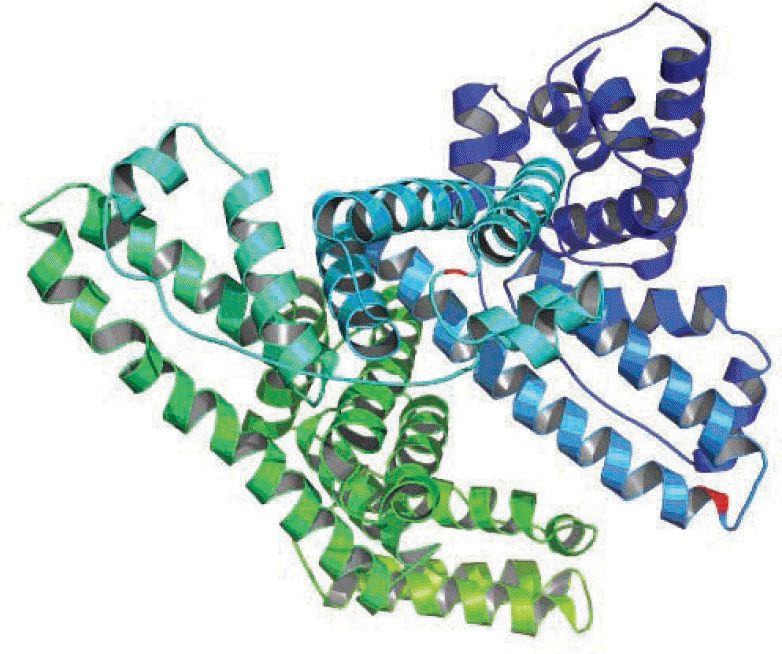 | Fig. 3.Ribbon diagram of a monomer of human albumin of Korean patients with bisalbuminemia; the locations of the amino acid replacements are depicted. The substituted amino acid residues are indicated in red. |
Table 1.
Biochemical analysis of the components that show a binding affinity to albumin




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download