Abstract
Background:
Continuous monitoring systems have allowed determination of the time-to-positivity (TTP). We evaluated the clinical relevance of TTP in the BACTEC9240 system (Becton-Dickinson, USA).
Methods:
A total of 2,354 vials of positive blood cultures were evaluated over 2 months. TTP was monitored from each of BACTEC Plus Aerobic/F (BD) or Pediatric Plus/F and Lytic Anaerobic/F bottles, and the differential time-to-positivity (DTP) for blood samples drawn simultaneously via catheter and a peripheral site was determined.
Results:
The average TTP of the positive vials was 17.4 hr, and 79.9% and 95.2% of the vials showed positivity within 24 and 48 hr, respectively. While the average TTP values for Aeromonas hydrophila, Bacillus cereus, Acinetobacter baumannii, and Streptococcus pneumoniae were less than 10 hr, those for Candida spp., anaerobes, Propionibacterium acnes, Corynebacterium spp, Bacillus spp. other than cereus, and coagulase-negative staphylococci were 35.3, 27.0, 56.8, 45.8, 23.0, and 26.3 hr, respectively. The negative predictive values of TTP over 24 hr to predict Staphylococcus aureus among staphylococci and S. pneumoniae among α-hemolytic streptococci were 76.7% and 100%, respectively. Enterobacteriaceae and Enterococcus faecalis showed shorter TTP in anaerobic vials than in aerobic vials. DTP of more than 2 hr was observed for 27.8%, 72.2%, and 45.5% of S. aureus, S. epidermidis, and Candida spp.
Conclusions:
TTP can be used to discriminate pathogens and contaminants. The shorter TTP in anaerobic vials of certain Enterobacteriaceae and Enterococcus spp. would facilitate further identification. DTP is useful for diagnosing catheter-related bloodstream infection by S. aureus, S. epidermidis, and Candida spp.
Go to : 
REFERENCES
1.Clinical and Laboratory Standards Institute. Principles and procedures for blood cultures; approved guideline. M47-A. Wayne, PA: Clinical and Laboratory Standards Institute;2007.
2.Khatib R., Riederer K., Saeed S., Johnson LB., Fakih MG., Sharma M, et al. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin Infect Dis. 2005. 41:594–8.
3.Marra AR., Edmond MB., Forbes BA., Wenzel RP., Bearman GM. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. 2006. 44:1342–6.
4.Martinez JA., Soto S., Fabrega A., Almela M., Mensa J., Soriano A, et al. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J Clin Microbiol. 2006. 44:1468–74.
5.Fernandez J., Erstad BL., Petty W., Nix DE. Time to positive culture and identification for Candida blood stream infections. Diagn Microbiol Infect Dis. 2009. 64:402–7.
6.Peralta G., Rodriguez-Lera MJ., Garrido JC., Ansorena L., Roiz MP. Time to positivity in blood cultures of adults with Streptococcus pneumoniae bacteremia. BMC Infect Dis. 2006. 6:79.

7.Peralta G., Roiz MP., Sanchez MB., Garrido JC., Ceballos B., Rodriguez-Lera MJ, et al. Time-to-positivity in patients with Escherichia coli bacteraemia. Clin Microbiol Infect. 2007. 13:1077–82.
8.Blot F., Nitenberg G., Chachaty E., Raynard B., Germann N., Antoun S, et al. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet. 1999. 354:1071–7.

9.Raad I., Hanna HA., Alakech B., Chatzinikolaou I., Johnson MM., Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004. 140:18–25.

10.Souvenir D., Anderson DE Jr., Palpant S., Mroch H., Askin S., Anderson J, et al. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol. 1998. 36:1923–6.

11.Martinez JA., Pozo L., Almela M., Marco F., Soriano A., Lopez F, et al. Microbial and clinical determinants of time-to-positivity in patients with bacteraemia. Clin Microbiol Infect. 2007. 13:709–16.

12.Hernaiz C., Picardo A., Alos JI., Gomez-Garces JL. Nosocomial bacteremia and catheter infection by Bacillus cereus in an immunocompetent patient. Clin Microbiol Infect. 2003. 9:973–5.
13.Lee YL., Shih SD., Weng YJ., Chen C., Liu CE. Fatal spontaneous bacterial peritonitis and necrotizing fasciitis with bacteraemia caused by Bacillus cereus in a patient with cirrhosis. J Med Microbiol. 2010. 59:2424.

14.Choi JP., Lee SO., Kwon HH., Kwak YG., Choi SH., Lim SK, et al. Clinical significance of spontaneous Aeromonas bacterial peritonitis in cirrhotic patients: a matched case-control study. Clin Infect Dis. 2008. 47:66–72.
15.Ruimy R., Armand-Lefevre L., Andremont A. Short time to positivity in blood culture with clustered gram-positive cocci on direct smear examination is highly predictive of Staphylococcus aureus. Am J Infect Control. 2005. 33:304–6.
16.Weinstein MP., Towns ML., Quartey SM., Mirrett S., Reimer LG., Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997. 24:584–602.

17.Huh JW., Pai CH. Efficacy of routine anaerobic blood cultures. Korean J Clin Pathol. 1994. 14:161–7. (허정원및배직현. 혐기성혈액배양의중요성. 대한임상병리학회지 1994;14:161-7.).
18.Cockerill FR 3rd., Hughes JG., Vetter EA., Mueller RA., Weaver AL., Ilstrup DM, et al. Analysis of 281,797 consecutive blood cultures performed over an eight-year period: trends in microorganisms isolated and the value of anaerobic culture of blood. Clin Infect Dis. 1997. 24:403–18.

19.Horvath LL., Hospenthal DR., Murray CK., Dooley DP. Detection of simulated candidemia by the BACTEC 9240 system with plus aerobic/F and anaerobic/F blood culture bottles. J Clin Microbiol. 2003. 41:4714–7.

20.David A., Risitano DC., Mazzeo G., Sinardi L., Venuti FS., Sinardi AU. Central venous catheters and infections. Minerva Anestesiol. 2005. 71:561–4.
Go to : 
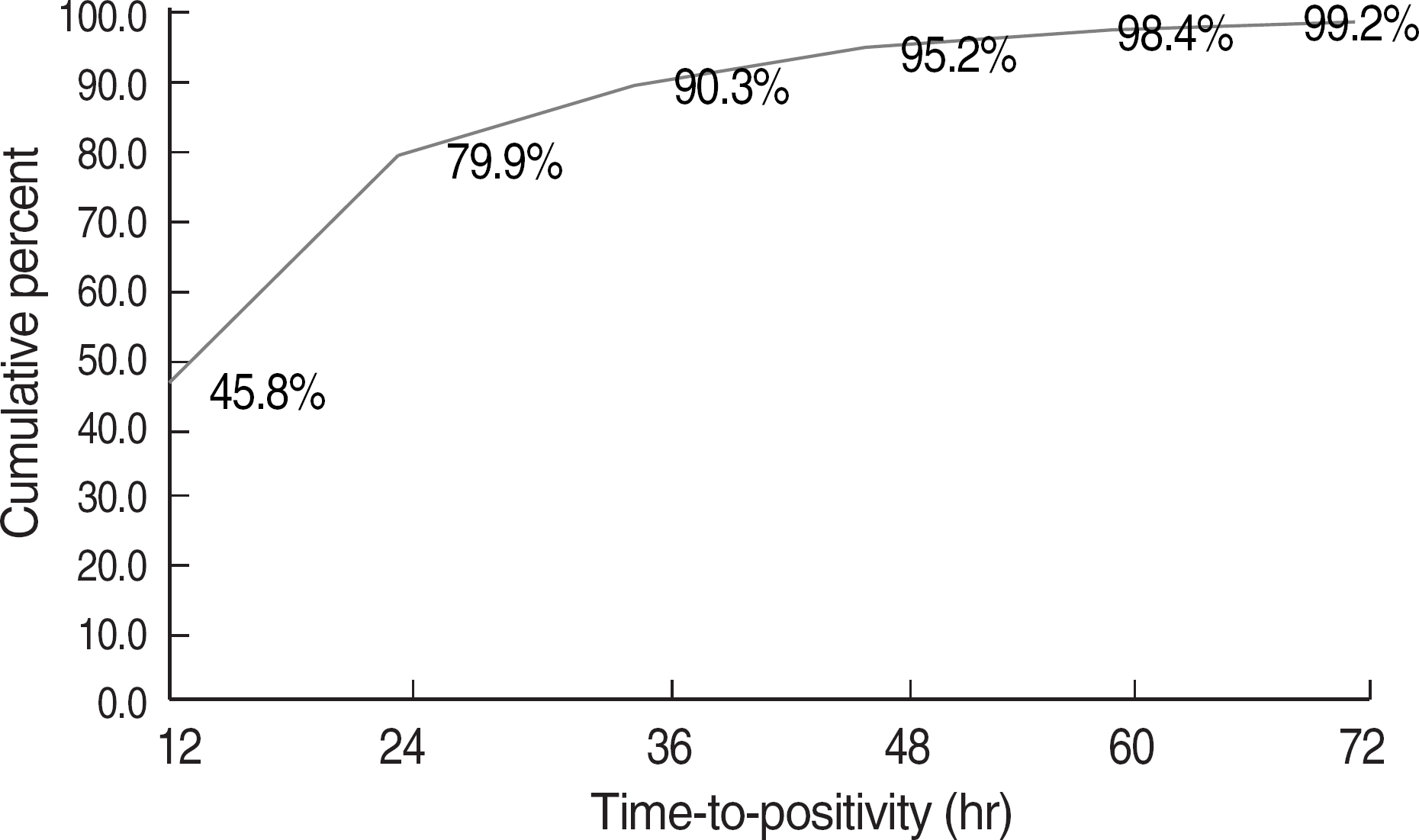 | Fig. 1.Cumulative percentages of positive blood culture vials at different time-to-positivity values. |
Table 1.
Time-to-positivity and number of positive vials of frequently isolated organisms
Table 2.
Comparison of time-to-positivity in 4 pairs of organisms
Table 3.
Time-to-positivity distributions of Staphylococcus aureus and CNS
Table 4.
Time-to-positivity of Enterobacteriaceae, Enterococcus spp., and Candida glabrata in aerobic and anaerobic vials
Table 5.
Differential time-to-positivity among frequent isolates of simultaneous peripheral and catheter culture




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download