Abstract
Background:
AML with myelodysplasia related changes (AML MRC) is known to show a poor prognosis compared with de novo AML, but controversies exist about the prognostic impact of multilineage dysplasia (MLD) among MRC. We investigated the prognostic impact of MLD in AML MRC.
Methods:
A total of 357 patients newly diagnosed as AML at Asan Medical Center from January 2001 to December 2005 were analyzed. They were diagnosed and classified as AML with recurrent genetic abnormalities, AML MRC, and AML not otherwise specified (AML NOS). Prognostic markers including overall survival (OS) and event free survival (EFS) were obtained through retrospective analysis of electronic medical records.
Results:
AML MRC patients showed a lower complete remission (CR) rate (44.7% vs. 64.9%, P= 0.002) and shorter OS (297 vs. 561 days, P=0.004) and EFS (229 vs. 374 days, P=0.004) than AML NOS patients. Patients with MLD among AML MRC also showed a lower CR rate (37.7%, P=0.001) and shorter OS (351 days, P=0.036) and EFS (242 days, P=0.076) than AML NOS patients. However, among AML MRC patients, there were no differences in OS, EFS and CR between patients with and without MLD.
Go to : 
REFERENCES
1.Swerdlow SH, Campo E, editors. WHO classification of tumours of hematopoietic and lymphoid tissues. 4th ed.Lyon: IARC Press;2008.
2.Gahn B., Haase D., Unterhalt M., Drescher M., Schoch C., Fonatsch C, et al. De novo AML with dysplastic hematopoiesis: cytogenetic and prognostic significance. Leukemia. 1996. 10:946–51.
3.Cunningham I., Gee TS., Reich LM., Kempin SJ., Naval AN., Clarkson BD. Acute promyelocytic leukemia: treatment results during a decade at Memorial Hospital. Blood. 1989. 73:1116–22.

4.Sobol RE., Mick R., Royston I., Davey FR., Ellison RR., Newman R, et al. Clinical importance of myeloid antigen expression in adult acute lymphoblastic leukemia. N Engl J Med. 1987. 316:1111–7.

5.Yunis JJ., Brunning RD., Howe RB., Lobell M. High-resolution chromosomes as an independent prognostic indicator in adult acute nonlymphocytic leukemia. N Engl J Med. 1984. 311:812–8.

6.Gerhartz HH., Schmetzer H. Detection of minimal residual disease in acute myeloid leukemia. Leukemia. 1990. 4:508–16.
7.Jaffe ES., Harris NL, et alWorld Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press. 2001.
8.Arber DA., Stein AS., Carter NH., Ikle D., Forman SJ., Slovak ML. Prognostic impact of acute myeloid leukemia classification. Importance of detection of recurring cytogenetic abnormalities and multi-lineage dysplasia on survival. Am J Clin Pathol. 2003. 119:672–80.
9.Haferlach T., Schoch C., Loffler H., Gassmann W., Kern W., Schnittger S, et al. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies. J Clin Oncol. 2003. 21:256–65.

10.Yanada M., Suzuki M., Kawashima K., Kiyoi H., Kinoshita T., Emi N, et al. Long-term outcomes for unselected patients with acute myeloid leukemia categorized according to the World Health Organization classification: a single-center experience. Eur J Haematol. 2005. 74:418–23.

11.Wandt H., Schakel U., Kroschinsky F., Prange-Krex G., Mohr B., Thiede C, et al. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood. 2008. 111:1855–61.

12.Jinnai I., Tomonaga M., Kuriyama K., Matsuo T., Nonaka H., Amenomori T, et al. Dysmegakaryocytopoiesis in acute leukaemias: its predominance in myelomonocytic (M4) leukaemia and implication for poor response to chemotherapy. Br J Haematol. 1987. 66:467–72.

13.Brito-Babapulle F., Catovsky D., Galton DA. Clinical and laboratory features of de novo acute myeloid leukaemia with trilineage myelodysplasia. Br J Haematol. 1987. 66:445–50.

14.Kuriyama K., Tomonaga M., Matsuo T., Kobayashi T., Miwa H., Shirakawa S, et al. Poor response to intensive chemotherapy in de novo acute myeloid leukaemia with trilineage myelodysplasia. Japan Adult Leukaemia Study Group (JALSG). Br J Haematol. 1994. 86:767–73.
15.Kim JM., Seo EJ., Park CJ., Chi HS. Significance of trilineage myelodysplasia in de novo acute myeloid leukemia. Korean J Clin Pathol. 2000. 20:442–8. (김지명, 서을주, 박찬정, 지현숙. 급성골수성백혈병에서삼계열이형성의의의. 대한임상병리학회지 2000;20:442-8.).
16.Hoyle CF., Gray RG., Wheatley K., Swirsky D., de Bastos M., Sherrington P, et al. Prognostic importance of Sudan Black positivity: a study of bone marrow slides from 1,386 patients with de novo acute myeloid leukaemia. Br J Haematol. 1991. 79:398–407.

17.Lima CS., Vassalo J., Lorand-Metze I., Bechelli AP., Souza CA. The significance of trilineage myelodysplasia in de novo acute myeloblastic leukemia: clinical and laboratory features. Haematologia (Budap). 1997. 28:85–95.
18.Weinberg OK., Seetharam M., Ren L., Seo K., Ma L., Merker JD, et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood. 2009. 113:1906–8.

19.Bao L., Wang X., Ryder J., Ji M., Chen Y., Chen H, et al. Prospective study of 174 de novo acute myelogenous leukemias according to the WHO classification: subtypes, cytogenetic features and FLT3 mutations. Eur J Haematol. 2006. 77:35–45.
Go to : 
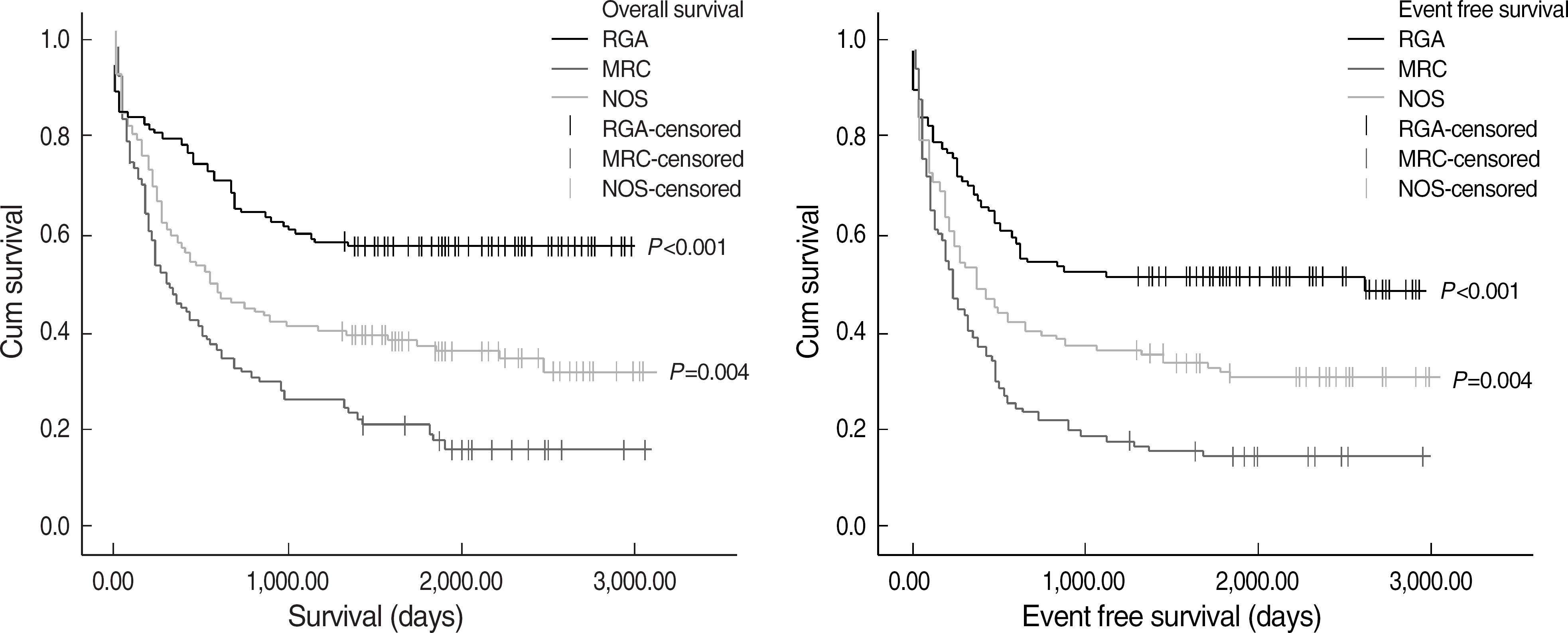 | Fig. 1.Comparison of overall survival and event free survival in 357 patients among 3 AML groups according to revised 2008 WHO classification. Abbreviations: RGA, recurrent genetic abnormalities; MRC, myelodysplasia related changes; NOS, not otherwise specified. |
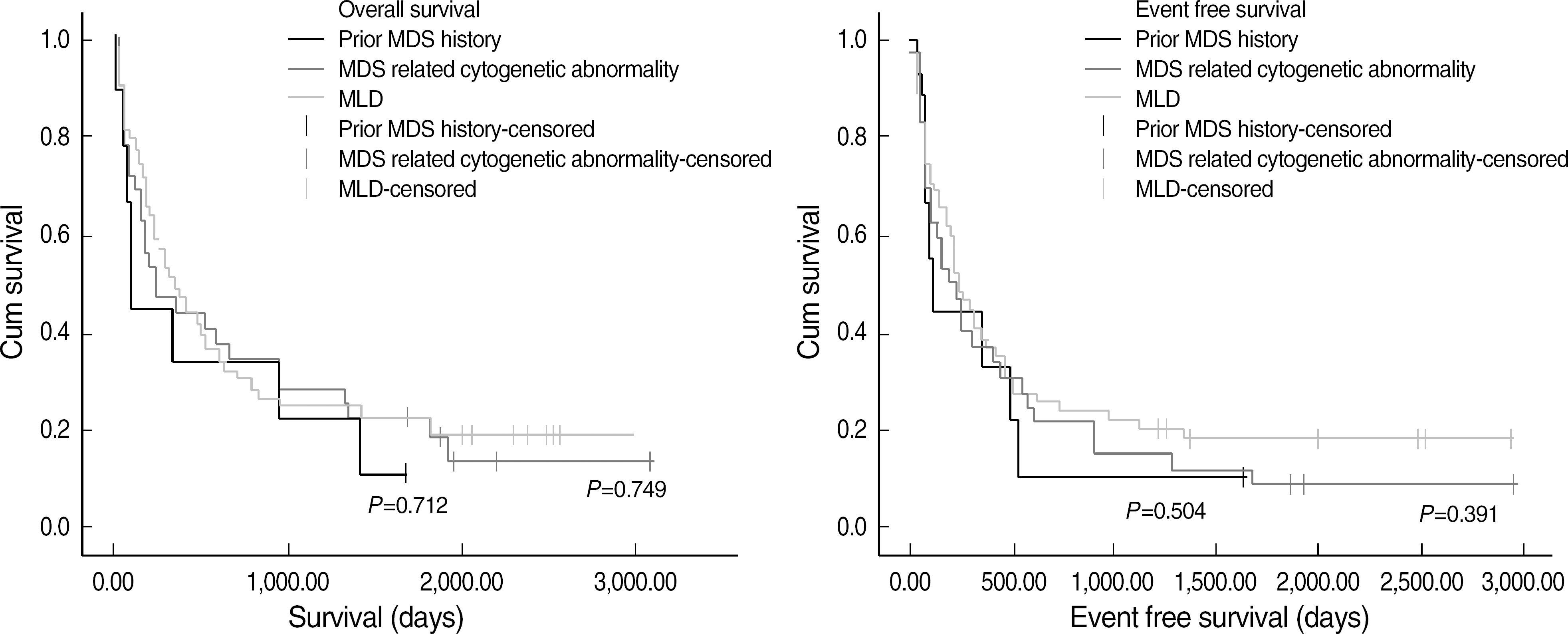 | Fig. 2.Fig. 2. Comparison of overall survival and event free survival of AML MRC patients among 3 subgroups. Abbreviation: MLD, multilineage dysplasia. |
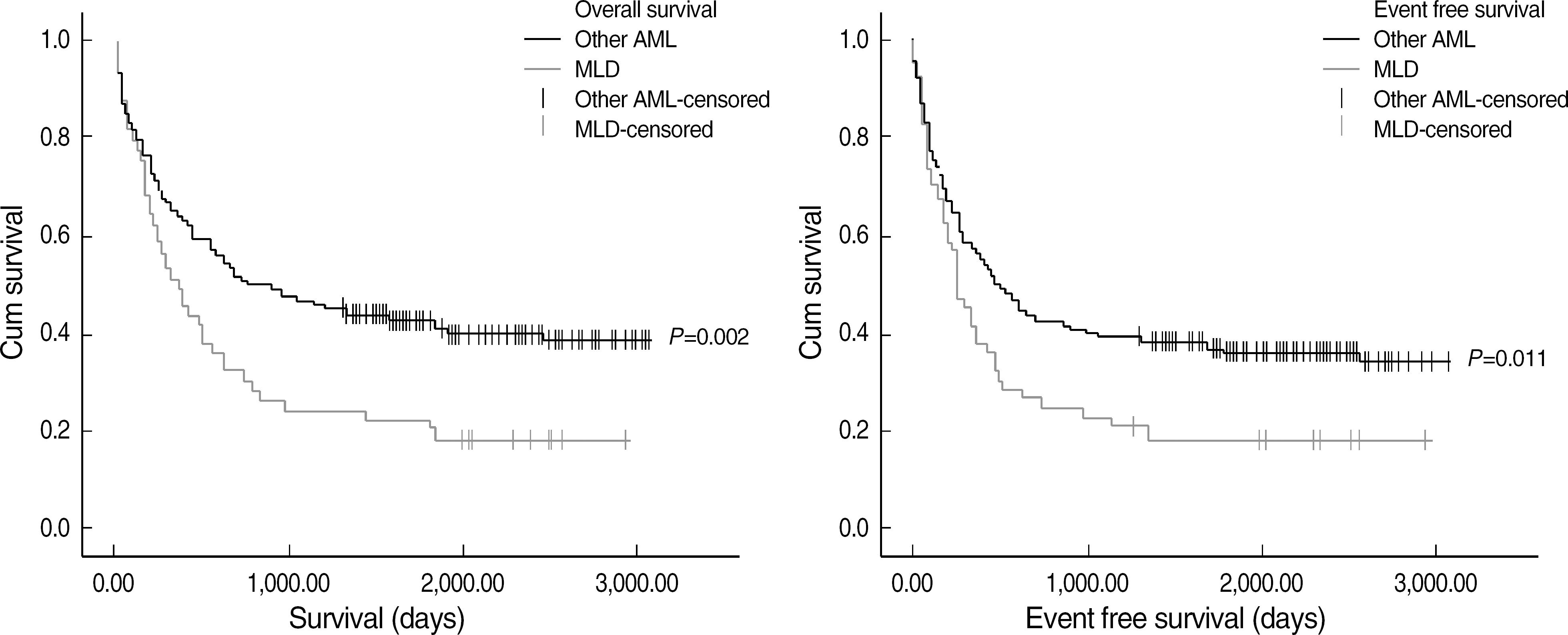 | Fig. 3.Comparison of overall survival and event free survival of AML MLD patients compared with other AML. Abbreviation: MLD, multilineage dysplasia. |
Table 1.
Clinical characteristics and prognosis in 3 AML groups
| Clinical variables | AML RGA (N=115) | AML NOS (N=148) | AML MRC (N=94) | P value∗ |
|---|---|---|---|---|
| Age, median (range) | 42 (2-80) | 45 (1-82) | 53 (2-79) | 0.362 |
| Hb (g/dL), median (range) | 8.8 (2.6-14.7) | 9.0 (3.6-15.1) | 8.5 (3.2-11.6) | 0.09 |
| WBC (×1,000 /μL), median (range) | 8.4 (0.4-239.4) | 10.8 (0.7-333.4) | 5.1 (0.3-246.0) | <0.001 |
| Platelet (×1,000 /μL), median (range) | 44.0 (5.0-511.0) | 57.0 (2.9-302.0) | 42.5 (6.0-433.0) | 0.992 |
| Blast (%), median (range) | 66.8 (20.2-96.2) | 66.1 (5.2-98.8) | 50.5 (16.4-96.2) | <0.001 |
| FLT3 ITD mutation | 100.0% | 17.4% | 5.1% | 0.011 |
| FLT3 D835 mutation | 14.7% | 7.6% | 5.0% | 0.605 |
| CR | 79.1% | 64.9% | 44.7% | 0.002 |
| Relapse | 26.1% | 30.4% | 29.8% | 0.919 |
| Death | 26.1% | 64.9% | 76.6% | 0.054 |
| OS (days), median | 1,455.0, P<0.001† | 561 | 297 | 0.004 |
| EFS (days), median | 1,294.0, P<0.001† | 374 | 229 | 0.004 |
Table 2.
Comparison of OS, EFS, CR and death rate among subgroups of AML MRC
Table 3.
Comparison of OS, EFS, CR and death rate between MLD and other MRC
| Median (days) | CR | Death | ||
|---|---|---|---|---|
| OS | EFS | |||
| MLD | 351.0 | 242.0 | 37.7% | 81.1% |
| (P value compared with other MRC) | P=0.342 | P=0.154 | P=0.124 | P=0.238 |
| Other MRC | 212.0 | 162.0 | 53.7% | 70.7% |
Table 4.
Comparison of OS, EFS, CR and death rate between AML MRC and other AML
Table 5.
Comparison of OS, EFS, CR and death rate between AML MLD and other AML
Table 6.
Factors associated with OS, EFS, CR and relapse rate




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download