Abstract
Background:
Methicillin-resistant Staphylococcus aureus (MRSA) may cause infections during wound dressing. We aimed to compare the antibacterial activities and wound-healing effects of commercially available silver-coated or silver-impregnated wound dressings on MRSA-infected wounds.
Methods:
Full-thickness skin defects were made on the back of rats (N=108) and were infected with MRSA. The rats were divided into the following 6 groups according to the dressing used for the wounds: nanocrystalline silver (Acticoat®), silver carboxymethylcellulose (Aquacel®-Ag), silver sulfadiazine (Medifoam silver®), nanocrystalline silver (PolyMem silver®), silver sulfadiazine (Ilvadon®) and 10% povidone iodide (Betadine®). We analyzed the wound sizes, histological findings, and bacterial colony counts for the groups. We also inoculated the silver materials on Mueller-Hinton agar plates containing MRSA and compared the inhibition zones in the agar plates.
Results:
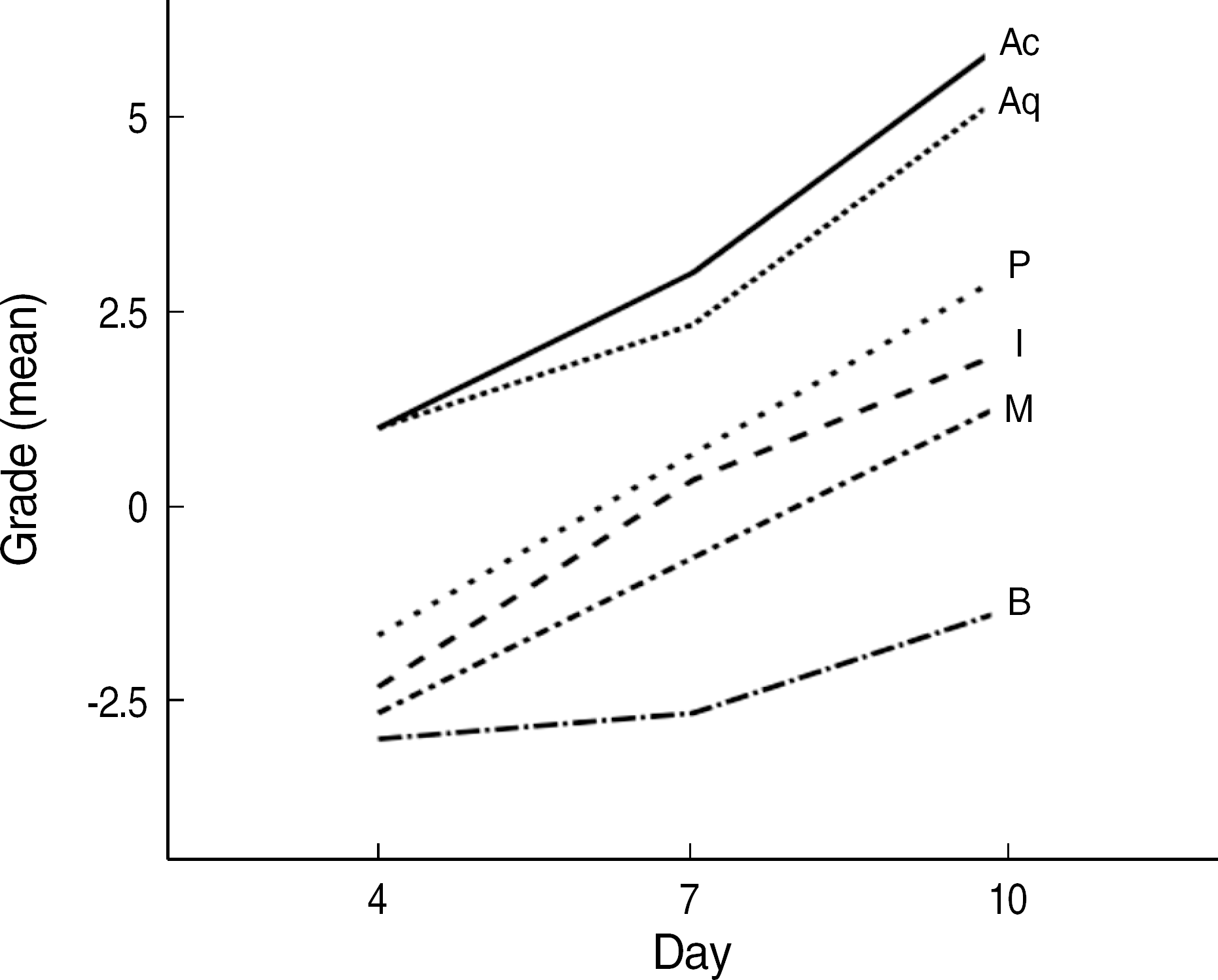
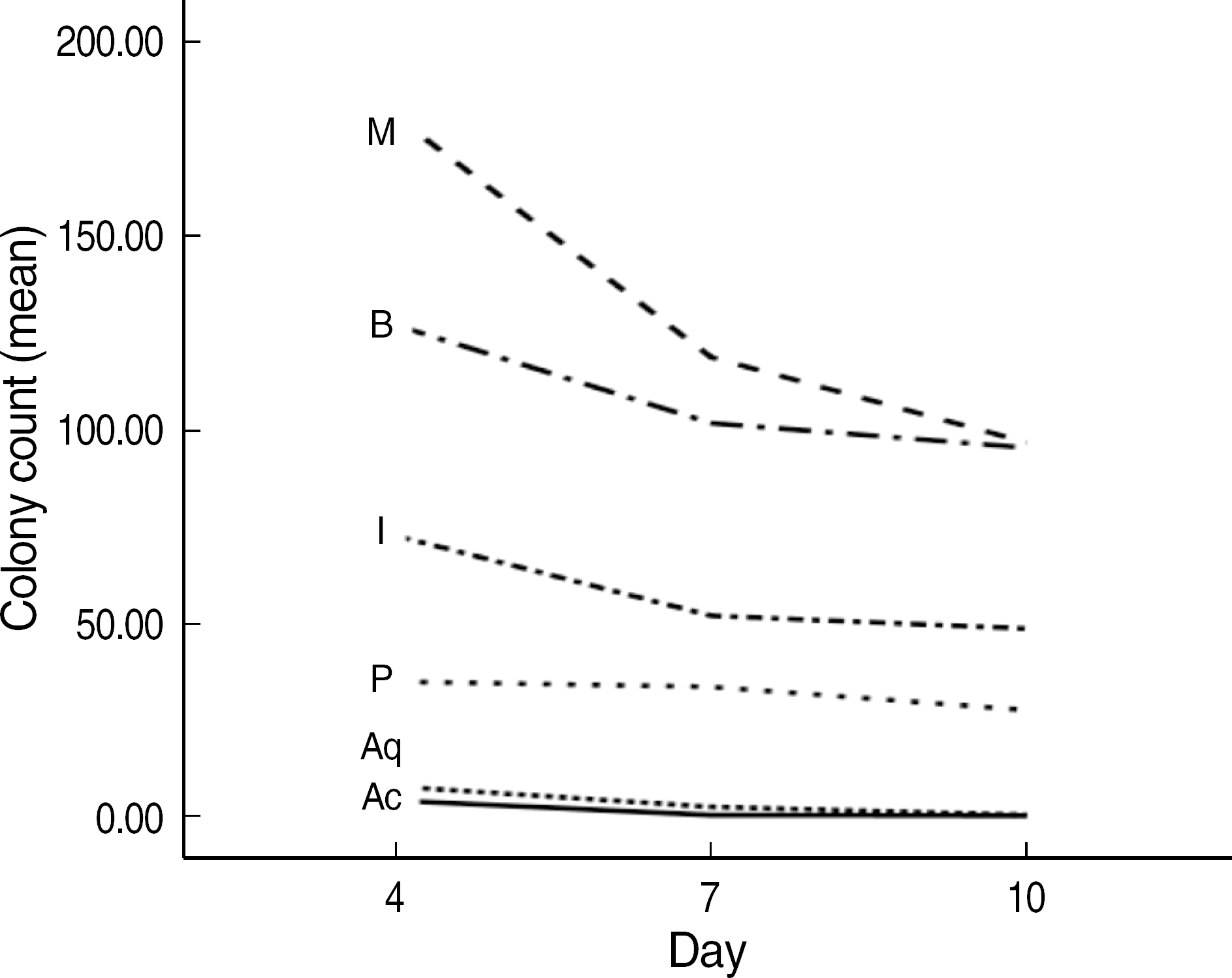
The order of the rate of wound-size decrease was Acticoat®>Aquacel®-Ag>PolyMem silver®>Medifoam silver®>Ilvadon®>Betadine®. The histological findings revealed that the Acticoat® showed more reepithelialization and granulation tissue formation and less inflammatory cell infiltration than the other materials. The order of the time required for wound healing was Acticoat®>Aquacel®-Ag>PolyMem silver®>Ilvadon®>Medifoam silver®>Betadine®. The bacterial colony counts reduced in all the groups, except in the Medifoam silver® group. The order of the size of the inhibition zone was Acticoat®>Aquacel®-Ag>Ilvadon®>PolyMem silver®>Betadine®>Medifoam silver®.
Conclusions:
Silver-coated or silver-impregnated wound dressings can be used for treating MRSA-infected wounds. Considering its superior efficacy in comparison to the efficacies of other silver-coated or silver-impregnated wound dressings, Acticoat® should be preferentially used for the treatment of MRSA-infected skin wounds.
Go to : 
REFERENCES
1.Kim HB., Jang HC., Nam HJ., Lee YS., Kim BS., Park WB, et al. In vitro activities of 28 antimicrobial agents against Staphylococcus aureus isolates from tertiary-care hospitals in Korea: a nationwide survey. Antimicrob Agents Chemother. 2004. 48:1124–7.
2.Hernandez R. The use of systemic antibiotics in the treatment of chronic wounds. Dermatol Ther. 2006. 19:326–37.

3.Attinger CE., Janis JE., Steinberg J., Schwartz J., Al-Attar A., Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006. 117(S7):72–109.
4.Jeong TK., Yang HJ. Effectiveness of nanocrystalline silver (Acticoat®) dressing at wound infected by multidrug resistant bacteria. J Korean Soc Plast Reconstr Surg. 2007. 34:691–6. (정광태및양호직. 다제 내성을지닌균주에감염된상처에서 nanocrystalline silver (Acticoat®) 드레싱의효용. 대한성형외과학회지 2007;34:691-6.).
5.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998. 339:520–32.
6.Kim JH., Park MC., Lee IJ., Park DH. The use of vacuum-assisted-closure therapy for the treatment of Methicillin-Resistant-Staphylococcus aureus infected wounds. J Korean Soc Plast Reconstr Surg. 2006. 33:632–6. (김주형, 박명철, 이일재, 박동하. 메치실린 저항 포도알균에감염된 창상 치료에 있어 음압요법의 의의. 대한성형외과학회지 2006;33:632-6.).
7.Hartman BJ., Tomasz A. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984. 158:513–6.
8.Lansdown AB. A review of the use of silver in wound care: facts and fallacies. Br J Nurs. 2004. 13(S6):6–19.

9.Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006. 33:17–34.

10.Jones SA., Bowler PG., Walker M., Parsons D. Controlling wound bio-burden with a novel silver-containing Hydrofiber® dressing. Wound Repair Regen. 2004. 12:288–94.
11.Bowler PG., Jones SA., Walker M., Parsons D. Microbicidal properties of a silver-containing Hydrofiber® dressing against a variety of burn wound pathogens. J Burn Care Rehabil. 2004. 25:192–6.
12.Supp AP., Neely AN., Supp DM., Warden GD., Boyce ST. Evaluation of cytotoxicity and antimicrobial activity of Acticoat® burn dressing for management of microbial contamination in cultured skin substitutes grafted to athymic mice. J Burn Care Rehabil. 2005. 26:238–46.
13.Dunn K., Edwards-Jones V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns. 2004. 30(S1):1–9.

14.Mooney EK., Lippitt C., Friedman J. Plastic surgery educational foundation DATA committee. Silver dressings. Plast Reconstr Surg. 2006. 117:666–9.
15.Weber RS., Hankins P., Limitone E., Callender D., Frankenthaler RM., Wolf P, et al. Split-thickness skin graft donor site management. A randomized prospective trial comparing a hydrophilic polyurethane absorbent foam dressing with a petrolatum gauze dressing. Arch Otolaryngol Head Neck Surg. 1995. 121:1145–9.

16.Wells MD., Kirn DS. A new method of skin-graft stabilization: the reston technique. Ann Plast Surg. 1995. 34:554–6.

17.Salisbury RE., Bevin AG., Dingeldein GP., Grisham J. A clinical and laboratory evaluation of a polyurethane foam: a new donor site dressing. Arch Surg. 1979. 114:1188–92.
18.Ha TS., Cho YS., Kim DH., Hur J., Chun W., Kim JH, et al. The clinical effectiveness of Medifoam-silver in burns. J Korean Burn Soc. 2007. 10:65–70. (하태순, 조용석, 김도헌, 허준, 전욱, 김종현등. 화상에서메디폼-실버(메디폼-S)의임상적유용성. 대한화상학회지 2007;10:65-70.).
19.Atiyeh BS., Costagliola M., Hayek SN., Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007. 33:139–48.

20.Caruso DM., Foster KN., Blome-Eberwein SA., Twomey JA., Herndon DN., Luterman A, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006. 27:298–309.

21.Parsons D., Bowler PG., Myles V., Jones S. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical, and chemical characteristics. Wounds. 2005. 17:222–32.
22.Catledge SA., Fries MD., Vohra YK., Lacefield WR., Lemons JE., Woodard S, et al. Nanostructured ceramics for biomedical implants. J Nanosci Nanotechnol. 2002. 2:293–312.

23.Chae CH., Kim JY., Kim MJ., Chung H., Kim SH., Oh HW, et al. The therapeutic effect of silver nanocrystalline ointment on TMJ capsulitis. J Kor Oral Maxillofac Surg. 2006. 32:262–6. (채창훈, 김좌영, 김미자,정훈, 김승호, 오현우등. 은나노연고가측두하악관절낭염의환자의치료에미치는영향에관한연구. 대한구강악안면외과학회지 2006;32: 262-6.).
Go to : 
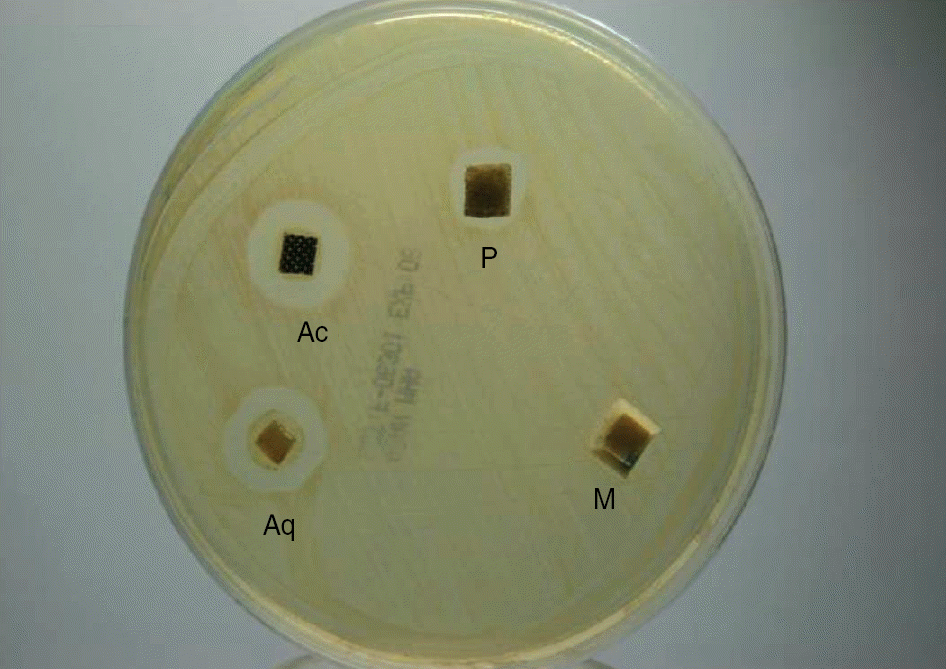 | Fig. 1.Antibacterial effect of silver-based dressing materials in Mueller-Hinton agar. The antibacterial effect was evaluated by measuring the growth-inhibition zones on the agar plate. After 24 hr, additional inhibition zones were observed along the edges of the dressing material.
Abbreviations: Ac, Acticoat®; Aq, Aquacel®-Ag; P, PolyMem silver®; M, Medifoam silver®.
|
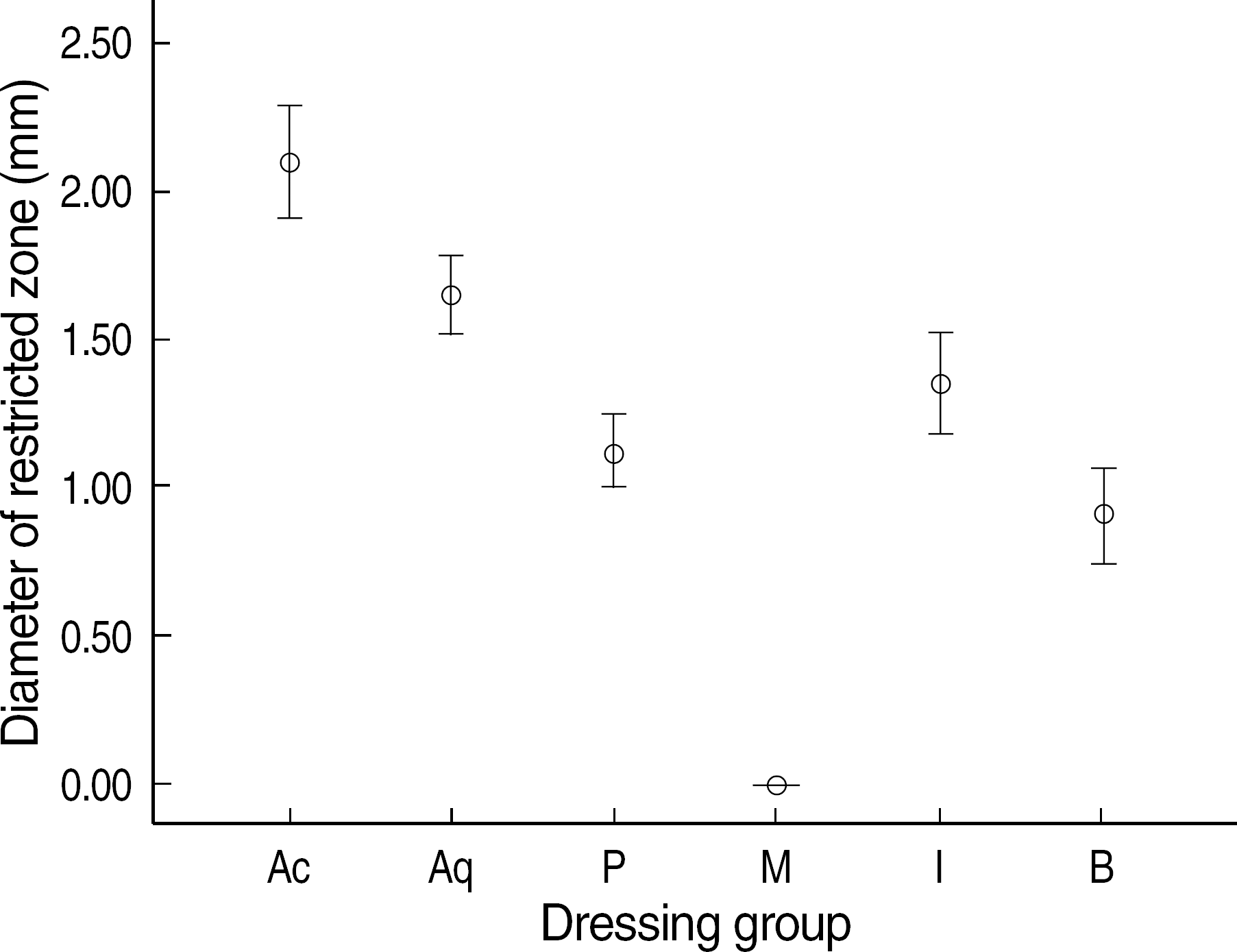 | Fig. 2.Error bars indicating the standard deviation for the antibacterial effects of silver dressing materials (Acticoat® [Ac], Aquacel®-Ag [Aq], PolyMem silver® [P], Medifoam silver® [M], Ilvadon® [I], and Betadine® [B] groups) in Mueller-Hinton agar. Statistically significant differences were observed among the groups (oneway ANOVA, P<0.001). |
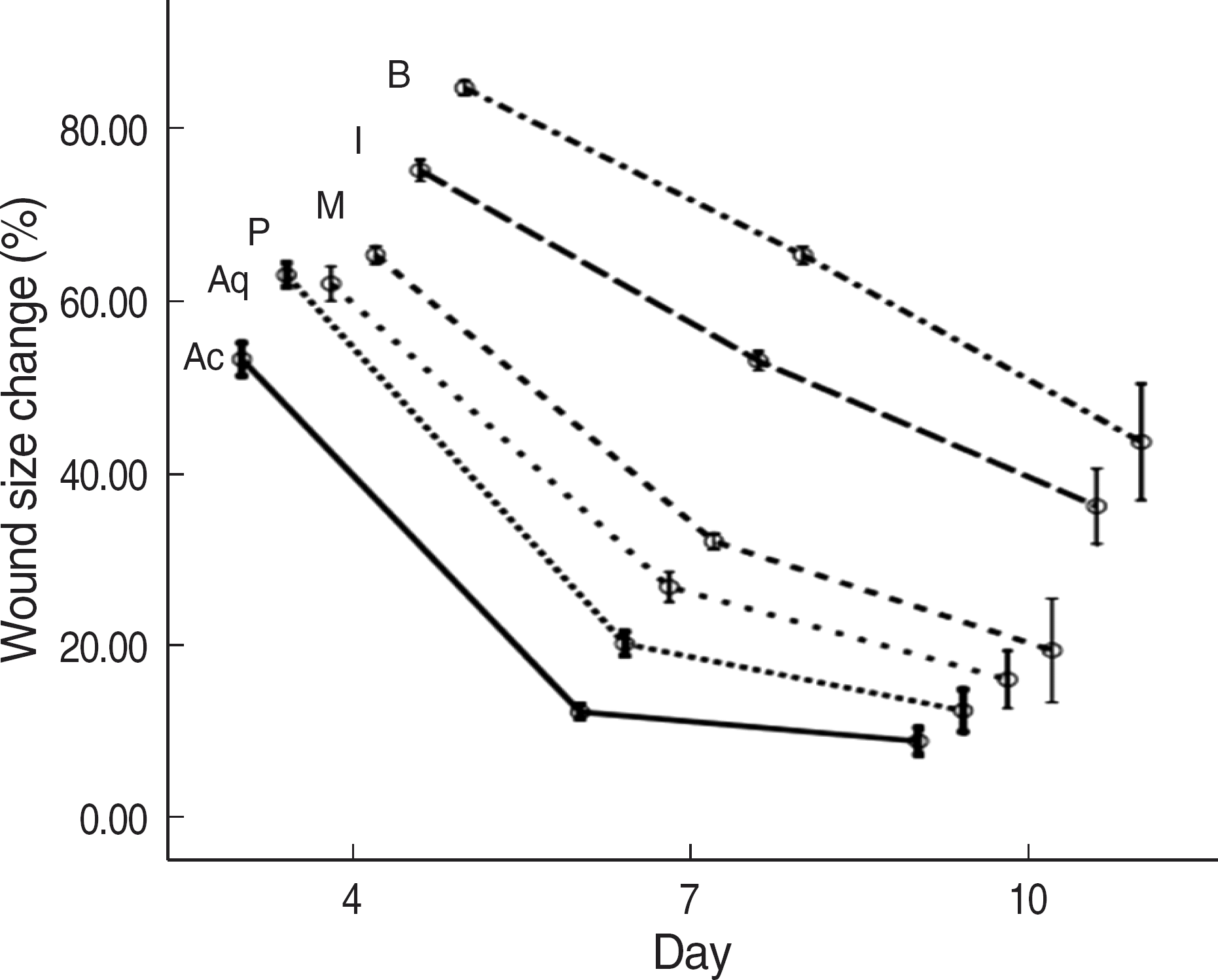 | Fig. 3.Error bars indicating the standard deviation for wound size change (%) according to the day and type of wound dressing (Acticoat® [Ac], Aquacel®-Ag [Aq], PolyMem silver® [P], Medifoam silver® [M], Ilvadon® [I], and Betadine® [B] groups). The differences among the groups were statistically significant (two-way ANOVA, P<0.001). |
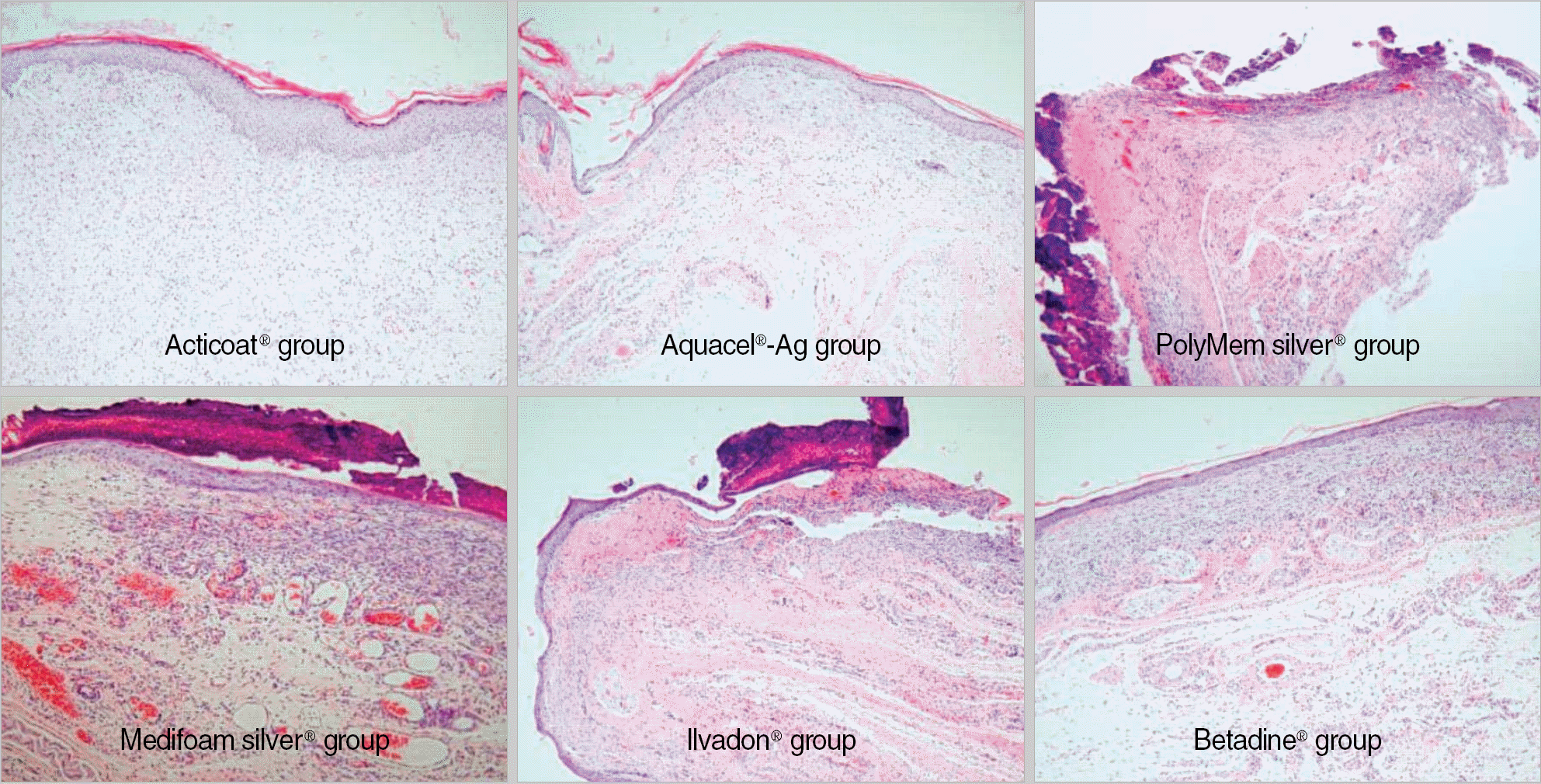 | Fig. 4.Histological findings in each group (hematoxylin and eosin stain, ×200) at 10 days . |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download