Abstract
Background:
We developed and evaluated the utility of a multiplex real-time PCR assay that uses melting curve analysis and allows simultaneous identification of vancomycin-resistant genotypes and clinically relevant enterococci.
Methods:
The specificity of the assay was tested using 4 reference strains of vancomycin-resistant enterococci (VRE) and 2 reference strains of vancomycin-susceptible enterococci. Ninety-three clinical isolates of enterococci with different glycopeptide-resistant phenotypes were genotyped and identified using a multiplex real-time PCR assay and melting curve analysis.
Results:
Representative melting curves were obtained for Enterococcus faecium, Enterococcus faecalis, vanA-containing E. faecium, vanB-containing E. faecalis, Enterococcus gallinarum, and Enterococcus casseliflavus. Phenotypic and genotypic analysis of the isolates revealed same results for 82 enterococcal isolates, while in 4 isolates, the glycopeptide-resistant phenotypes were inconsistent with the glycopeptide-resistant genotypes and in the 4 other isolates, species could not be accurately identified. Three isolates with mixed strains, which were detected by the PCR assay, could not be correctly identified using phenotypic methods.
Go to : 
REFERENCES
1.Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988. 319:157–61.

2.Willems RJ., Top J., van Santen M., Robinson DA., Coque TM., Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005. 11:821–8.
3.Park JW., Kim YR., Shin WS., Kang MW., Han KJ., Shim SI. Susceptibility tests of vancomycin-resistant enterococci. Korean J Infect Dis. 1992. 24:133–7. (박지원, 김양리, 신완식, 강문원, 한경원, 심상인. Vancomycin 내성 enterococci에대한감수성검사. 감염 1992;24:133-7.).
4.Lee WG., Jung MK., Kwak YS. Vancomycin-resistant enterococci: incidence, antimicrobial susceptibility, and resistance genotypes. Korean J Clin Pathol. 1998. 18:51–6. (이위교, 정민권, 곽연식. Vancomycin 내성 장구균의 분리율, 항균제 감수성 및 내성형에 관한 연구. 대한임상병리학회지 1998;18:51-6.).
5.Korean Society for Nosocomial Infection Control. Konis web-based reports & analysis program. http://konis.cdc.go.kr/sub/reports_icu.htm. (Updated on Jun. 2009.
6.Leven M. Molecular methods for the detection of antibacterial resistance genes. Lorian V, editor. Antibiotics in laboratory medicine. 5th ed.Philadelphia, PA: Lippincott Williams & Wilkins;2005. p. 509–31.
7.Clark NC., Cooksey RC., Hill BC., Swenson JM., Tenover FC. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993. 37:2311–7.

8.Dutka-Malen S., Evers S., Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995. 33:24–7.

9.Satake S., Clark N., Rimland D., Nolte FS., Tenover FC. Detection of vancomycin-resistant enterococci in fecal samples by PCR. J Clin Microbiol. 1997. 35:2325–30.

10.Kariyama R., Mitsuhata R., Chow JW., Clewell DB., Kumon H. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J Clin Microbiol. 2000. 38:3092–5.

11.Perez-Hernandez X., Mendez-Alvarez S., Claverie-Martin F. A PCR assay for rapid detection of vancomycin-resistant enterococci. Diagn Microbiol Infect Dis. 2002. 42:273–7.
12.Palladino S., Kay ID., Costa AM., Lambert EJ., Flexman JP. Real-time PCR for the rapid detection of vanA and vanB genes. Diagn Microbiol Infect Dis. 2003. 45:81–4.

13.Palladino S., Kay ID., Flexman JP., Boehm I., Costa AM., Lambert EJ, et al. Rapid detection of vanA and vanB genes directly from clinical specimens and enrichment broths by real-time multiplex PCR assay. J Clin Microbiol. 2003. 41:2483–6.
14.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: nineteenth informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute;2009.
15.Rozen S., Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. Misener S, Krawetz SA, editors. Bioinformatics methods and protocols. Totowa, NJ: Humana Press;2000. p. 365–86.

16.Shipley GL. An introduction to real-time PCR. Dorak MT, editor. Real-time PCR. Abingdon, Oxfordshire: Taylor & Francis;2006. p. 1–26.
17.Ririe KM., Rasmussen RP., Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997. 245:154–60.

18.Wittwer CT., Reed GH., Gundry CN., Vandersteen JG., Pryor RJ. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003. 49:853–60.

19.Henegariu O., Heerema NA., Dlouhy SR., Vance GH., Vogt PH. Multiplex PCR: critical parameters and step-by-step protocol. Biotechniques. 1997. 23:504–11.

20.Markoulatos P., Siafakas N., Moncany M. Multiplex polymerase chain reaction: a practical approach. J Clin Lab Anal. 2002. 16:47–51.

21.Wittwer CT., Herrmann MG., Gundry CN., Elenitoba-Johnson KS. Real-time multiplex PCR assays. Methods. 2001. 25:430–42.

22.Chang S., Sievert DM., Hageman JC., Boulton ML., Tenover FC., Downes FP, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003. 348:1342–7.
23.Boyd DA., Willey BM., Fawcett D., Gillani N., Mulvey MR. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel D-Ala-D-Ser gene cluster, vanL. Antimicrob Agents Chemother. 2008. 52:2667–72.
24.Hong KS., Kang ES., Lee MA. Investigation of prevalence of vancomycin-resistant enterococci and genotypes of glycopeptide resistance using polymerase chain reaction. Korean J Clin Pathol. 1998. 18:372–8. (홍기숙, 강은숙, 이미애. Vancomycin 내성 Enterococci의 빈도조사 및중합효소연쇄반응을 이용한 유전자형의 분석. 대한임상병리학회지 1998;18:372-8.).
25.Lee SY., Park JH., Park HS., Lee MA., Kang ES., Hong KS. Comparison of antimicrobial susceptibility testing methods to detect glycopeptide resistance in enterococci: E-test, Vitek, disk diffusion and agar dilution method. Korean J Clin Pathol. 2000. 20:301–7. (이수연,박진희, 박향숙, 이미애, 강은숙, 홍기숙. Glycopeptide 내성장구균의항균제 감수성 검사법 비교: E-test, Vitek, 디스크 확산법 및 한천 희석법.대한임상병리학회지 2000;20:301-7.).
26.Park JH., Lee SY., Lee MA., Chung WS. Investigation of risk factors for vancomycin-resistant enterococci (VRE) infection and colonization. Korean J Clin Pathol. 2000. 20:308–13. (박진희, 이수연, 이미애, 정화순. 반코마이신 내성 장구균 장내 군집 및 감염의 위험인자 분석. 대한임상병리학회지 2000;20:308-13.).
27.Kwon OG., Uh Y., Jang IH., Lee MK., Yoon KJ., Kim HY. Trend of isolation and genotypes of vancomycin-resistant enterococci isolated from tertiary care hospital in Wonju area. Korean J Clin Pathol. 2000. 20:486–93. (권오건, 어영, 장인호, 이미경, 윤갑준, 김효열. 원주지역 3차병원에서 분리된 vancomycin 내성 장구균의 분리 추이와 내성 유전자형. 대한임상병리학회지 2000;20:486-93.).
28.Navarro F., Courvalin P. Analysis of genes encoding D-alanine-D-alanine ligase-related enzymes in Enterococcus casseliflavus and Enterococcus flavescens. Antimicrob Agents Chemother. 1994. 38:1788–93.

29.Paule SM., Trick WE., Tenover FC., Lankford M., Cunningham S., Stosor V, et al. Comparison of PCR assay to culture for surveillance detection of vancomycin-resistant enterococci. J Clin Microbiol. 2003. 41:4805–7.

30.Sloan LM., Uhl JR., Vetter EA., Schleck CD., Harmsen WS., Manahan J, et al. Comparison of the Roche LightCycler vanA/vanB detection assay and culture for detection of vancomycin-resistant enterococci from perianal swabs. J Clin Microbiol. 2004. 42:2636–43.
31.Stamper PD., Cai M., Lema C., Eskey K., Carroll KC. Comparison of the BD GeneOhm VanR assay to culture for identification of vancomycin-resistant enterococci in rectal and stool specimens. J Clin Microbiol. 2007. 45:3360–5.

32.Sahm DF., Free L., Smith C., Eveland M., Mundy LM. Rapid characterization schemes for surveillance isolates of vancomycin-resistant enterococci. J Clin Microbiol. 1997. 35:2026–30.

33.Petrich A., Luinstra K., Page B., Callery S., Stevens D., Gafni A, et al. Effect of routine use of a multiplex PCR for detection of vanA- and vanB- mediated enterococcal resistance on accuracy, costs and earlier reporting. Diagn Microbiol Infect Dis. 2001. 41:215–20.

34.Drews SJ., Johnson G., Gharabaghi F., Roscoe M., Matlow A., Tellier R, et al. A 24-hour screening protocol for identification of vancomycin-resistant Enterococcus faecium. J Clin Microbiol. 2006. 44:1578–80.
35.Angeletti S., Lorino G., Gherardi G., Battistoni F., De Cesaris M., Dicuonzo G. Routine molecular identification of enterococci by gene-specific PCR and 16S ribosomal DNA sequencing. J Clin Microbiol. 2001. 39:794–7.

36.Song JH., Ko KS., Oh WS., Park S., Heo ST., Kwon KT, et al. High frequency of vancomycin-resistant Enterococcus faecium isolates with VanB phenotype and vanA genotype in Korean hospitals. Diagn Microbiol Infect Dis. 2006. 56:401–6.

37.Park IJ., Lee WG., Shin JH., Lee KW., Woo GJ. VanB phenotype-vanA genotype Enterococcus faecium with heterogeneous expression of teicoplanin resistance. J Clin Microbiol. 2008. 46:3091–3.
Go to : 
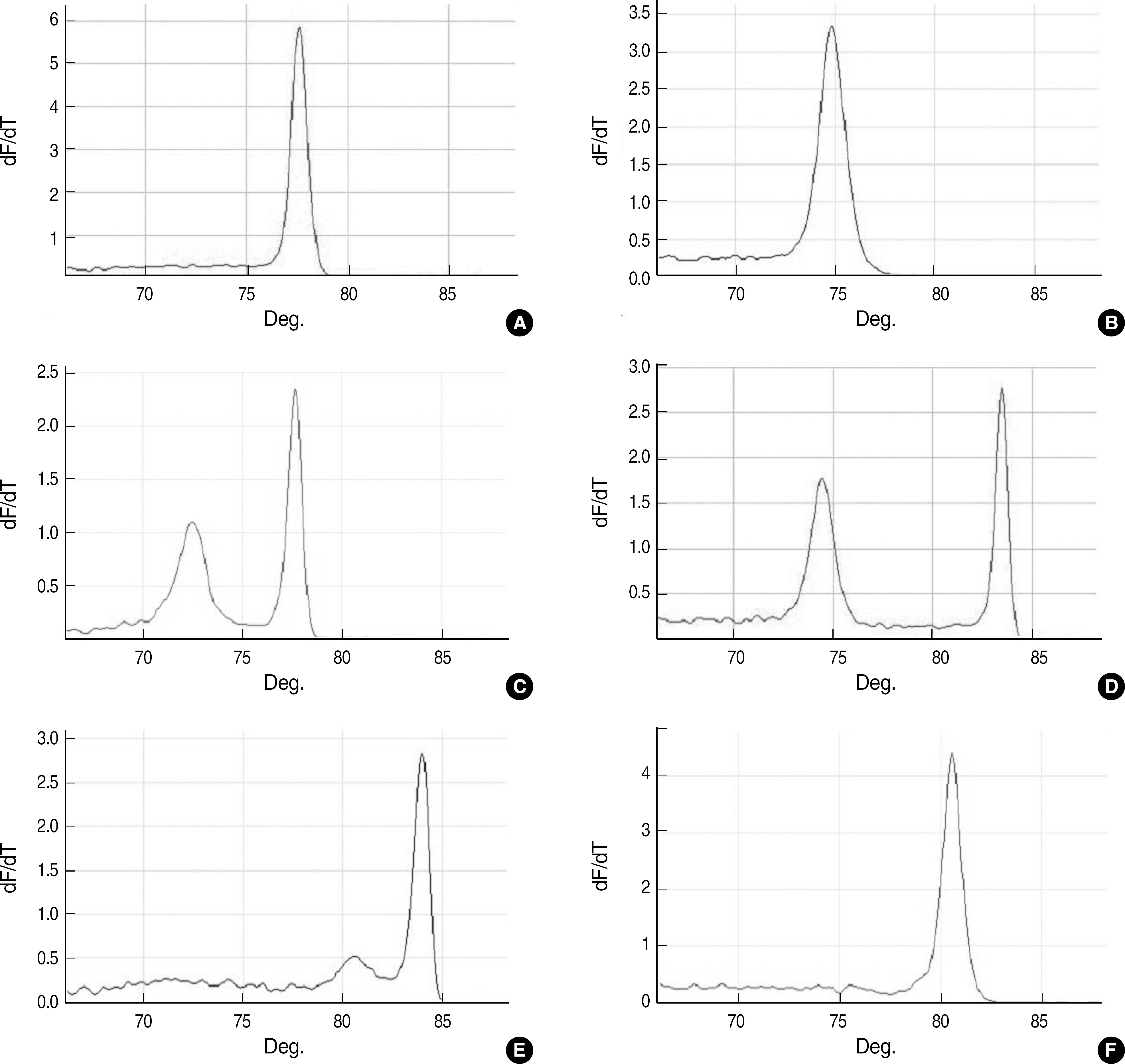 | Fig. 1.Melting curve analysis of amplicons obtained from reference strains by using multiplex real-time PCR. Melting curves for Enterococcus faecium (A); Enterococcus faecalis (B); E. faecium/vanA (C); E. faecalis/vanB (D); Enterococcus gallinarum/vanC1 (E); and Enterococcus casseliflavus/vanC2/C3 (F) are shown. |
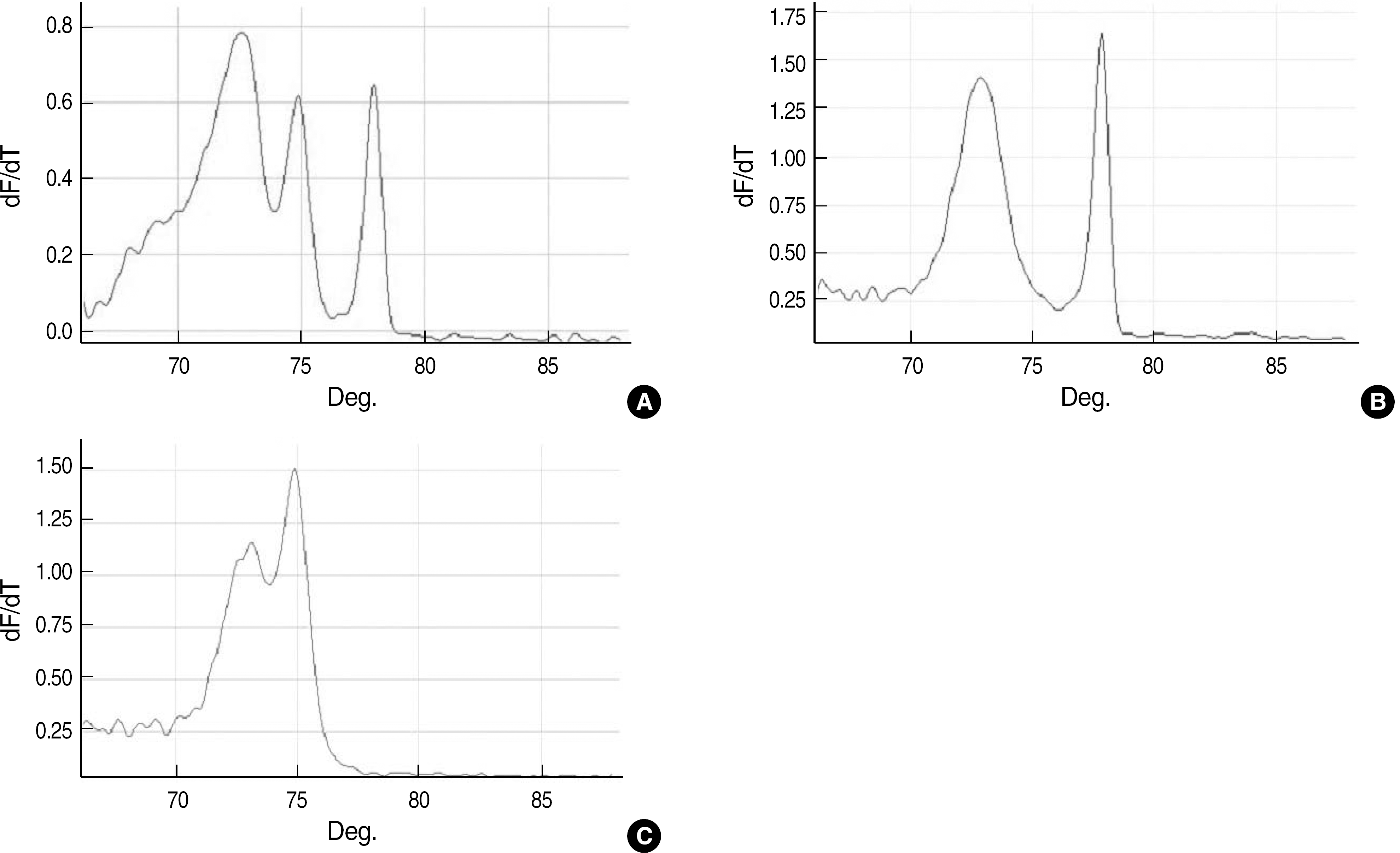 | Fig. 2.Melting curve analysis for the identification of mixed strains. (A) Melting curve for DNA extracted from black colonies growing on the Enterococcal agar plate. VanA, ddl Enterococcus faecalis, and ddl Enterococcus faecium peaks are shown. (B) Melting curve for DNA extracted from pure colonies of E. faecium with phenotype VanA growing on the blood agar plate. Peaks for E. faecium with VanA and ddl genes. (C) Melting curve for DNA extracted from pure colonies of E. faecalis with phenotype VanA growing on the blood agar plate. Peaks for E. faecalis with VanA and ddl genes. |
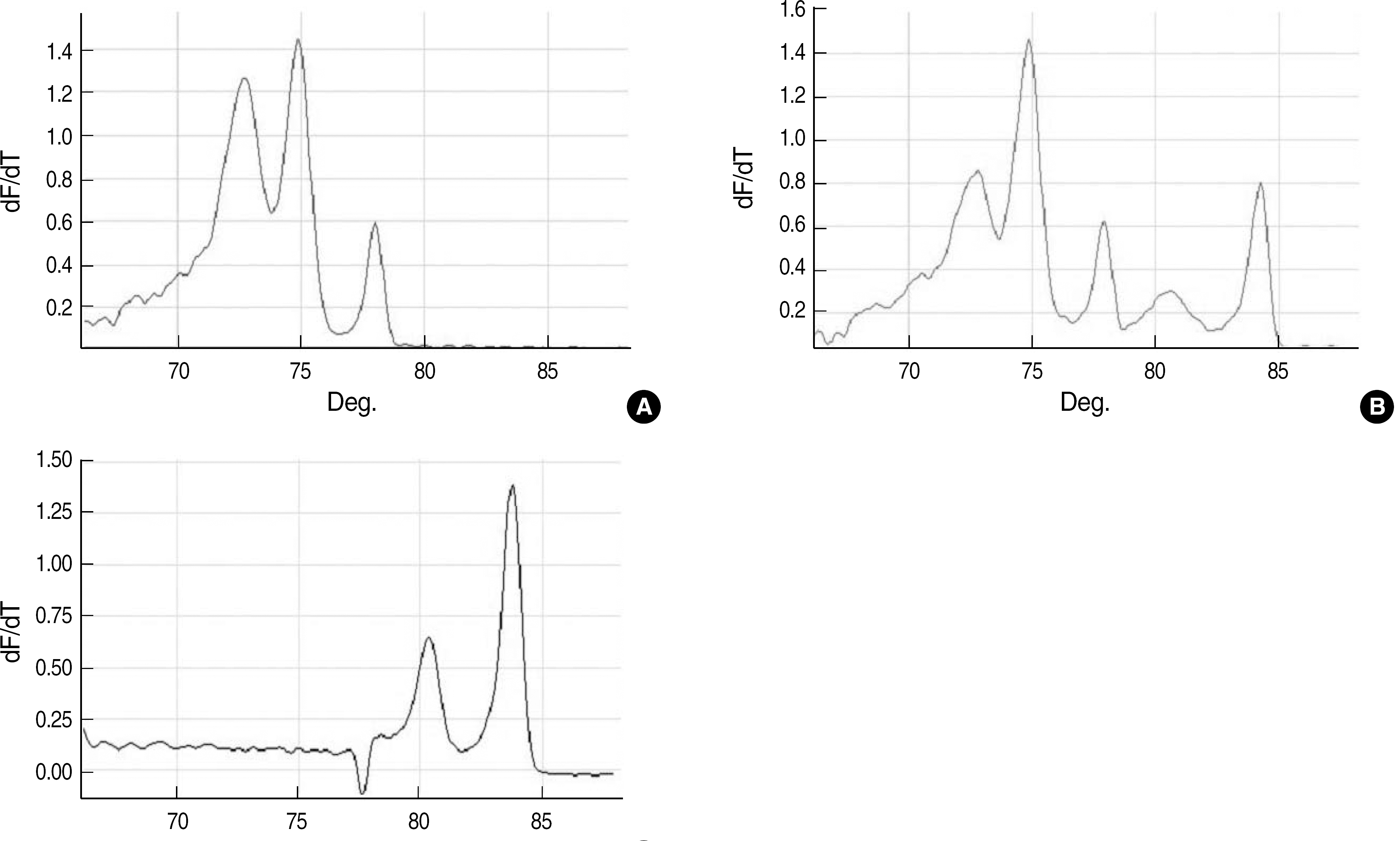 | Fig. 3.Melting curve analysis of amplicons obtained from 3 mixed strains of 2 or more enterococci by using multiplex real-time PCR. Melting curves for Enterococcus faecalis and Enterococcus faecium with vanA gene (A); E. faecalis and E. faecium with vanA gene and Enterococcus gallinarum with vanC1 gene (B); and Enterococcus casseliflavus/Enterococcus flavescens with vanC2/C3 gene and E. gallinarum with vanC1 gene (C). |
Table 1.
Multiplex real-time PCR primers for the detection of vancomycin-resistant enterococci
Table 2.
Genotype analysis of 93 enterococcal isolates with different glycopeptides-resistant phenotypes by using multiplex real-time PCR and melting curve analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download