Abstract
Background:
Accurate measurement of blood glucose concentrations is essential for defining diabetes, and the minimization of ex vivo glycolysis has been recommended. Recent guidelines advocate two kinds of methods for sample collection and processing: either the sodium fluoride (NaF) method or immediate refrigeration using a serum separation tube (SST). We investigated the difference between the two methods in measuring subsequent glucose concentrations using blood specimens from participants recruited for the fourth Korean National Health and Nutrition Examination Survey.
Methods:
Paired venous blood samples were collected in an SST and a NaF tube from 1,103 men and women. SST serum was separated within 30 min, including standing for 15 min, and then refrigerated. The NaF samples were refrigerated, but not separated until immediately before analysis. We compared the blood glucose concentrations between the SST (SST glucose) and NaF (NaF glucose) methods.
Results:
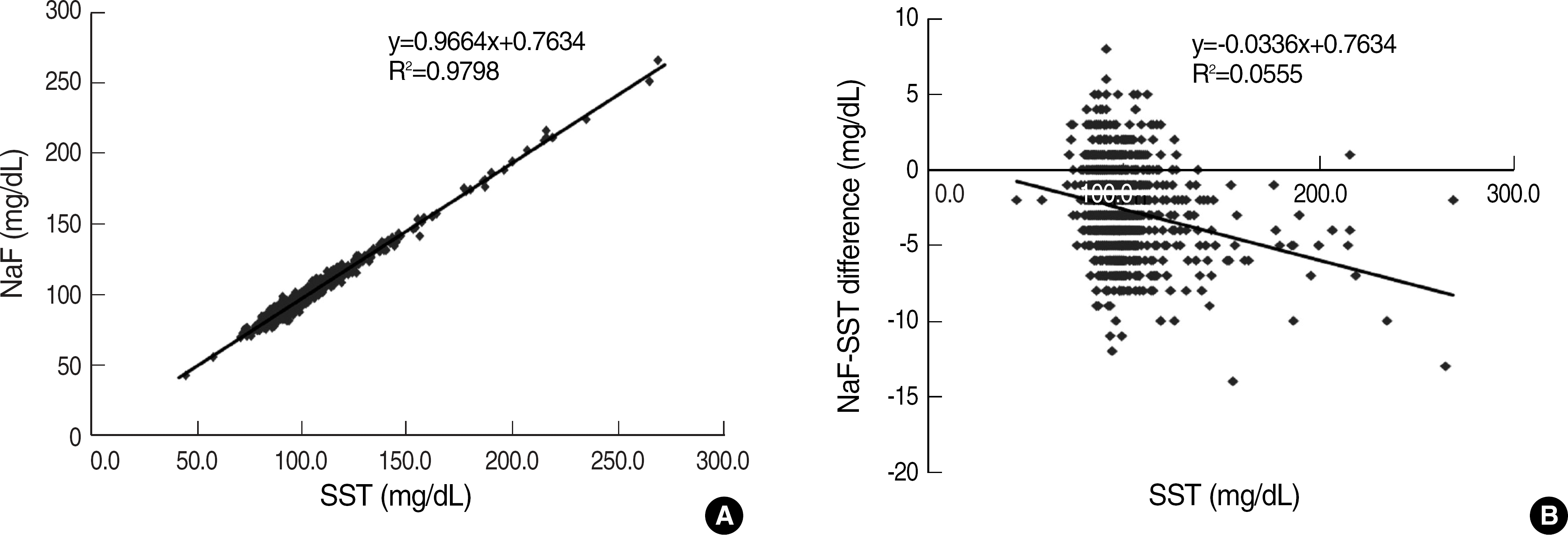
The mean SST glucose was significantly higher than NaF glucose (99.0 mg/dL vs 96.5 mg/dL, P<0.05). NaF glucose showed a negative mean bias of 2.6 mg/dL vs SST glucose but showed high correlation (R=0.9899). There was no significant correlation between the bias of blood glucose concentrations by two methods and the storage time of NaF glucose.
Conclusions:
The negative bias associated with the use of NaF tubes may significantly affect the prevalence of diabetes. Serum separation and refrigeration within 30 min after venous sampling is recommended over NaF method, not only to minimize the preanalytical impact on detecting diabetes but also to reduce sample volume and number of tubes.
REFERENCES
1.Sacks DB., Bruns DE., Goldstein DE., Maclaren NK., McDonald JM., Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002. 48:436–72.

2.Genuth S., Alberti KG., Bennett P., Buse J., Defronzo R., Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003. 26:3160–7.
3.le Roux CW., Wilkinson SD., Pavitt DV., Muller BR., Alaghband-Zadeh J. A new antiglycolytic agent. Ann Clin Biochem. 2004. 41:43–6.

4.Chan AY., Swaminathan R., Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989. 35:315–7.

5.Liss E., Bechtel S. Improvement of glucose preservation in blood samples. J Clin Chem Clin Biochem. 1990. 28:689–90.
6.Clark ML., Humphreys SM., Frayn KN. Stability of plasma glucose during storage. Ann Clin Biochem. 1990. 27(Pt 4):373–7.

7.Lee JK. Korea National Health and Nutrition Examination Survey. http://knhanes.cdc.go.kr. (Updated on May. 2009.
8.Waring WS., Evans LE., Kirkpatrick CT. Glycolysis inhibitors negatively bias blood glucose measurements: potential impact on the reported prevalence of diabetes mellitus. J Clin Pathol. 2007. 60:820–3.

9.Christopher MM., O'Neill S. Effect of specimen collection and storage on blood glucose and lactate concentrations in healthy, hyperthyroid and diabetic cats. Vet Clin Pathol. 2000. 29:22–8.
10.Mikesh LM., Bruns DE. Stabilization of glucose in blood specimens: mechanism of delay in fluoride inhibition of glycolysis. Clin Chem. 2008. 54:930–2.

11.Zhang DJ., Elswick RK., Miller WG., Bailey JL. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem. 1998. 44:1325–33.

12.Stahl M., Jorgensen LG., Hyltoft Petersen P., Brandslund I., de Fine Olivarius N., Borch-Johnsen K. Optimization of preanalytical conditions and analysis of plasma glucose. 1. Impact of the new WHO and ADA recommendations on diagnosis of diabetes mellitus. Scand J Clin Lab Invest. 2001. 61:169–79.
Fig. 1.
(A) Linear regression analysis of NaF method on SST method for blood glucose concentrations (mg/dL). (B) Absolute difference plot showing blood glucose concentrations from NaF method minus SST method (Y axis) versus SST method (X axis).
Abbreviations: NaF, sodium fluoride; SST, serum separation tube.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download