Abstract
Background:
Warfarin is a widely used oral anticoagulant with broad within- and between-individual dose requirements. Warfarin concentrations can be monitored by assessing its pharmacologic effects on International Normalized Ratio (INR). However, this approach has not been applied in the routine clinical management of patients receiving warfarin therapy. We performed a plasma warfarin assay using high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) to determine if such an assay can be utilized in routine clinical practice.
Methods:
We included a total of 105 patients with atrial fibrillation, and who were receiving warfarin for more than 1 yr. The plasma concentrations of total warfarin and 7-hydroxywarfarin were determined by HPLC-MS/MS (Waters, UK). We assessed the association between warfarin dose, concentration, and INR as well as the effects of these factors on warfarin concentrations.
Results:
The mean maintenance dose of warfarin in 105 patients was 4.1±1.3 mg/day (range, 1.7-8.0 mg/day) and their mean plasma warfarin concentration was 1.3±0.5 mg/L. We defined a concentration range of 0.6-2.6 mg/L (corresponding to the 2.5th to 97.5th percentile range of the Plasma warfarin levels in the 74 patients showing INR within target range) as the therapeutic range for warfarin. The correlation of warfarin dose with warfarin concentration (r2=0.259, P<0.001) was higher than that with INR (r2=0.029, P=0.072).
Conclusions:
There was a significant correlation between warfarin dose and plasma warfarin concentrations in Korean patients with atrial fibrillation. Hence, plasma warfarin monitoring can help determine dose adjustments and improve our understanding of individual patient response to warfarin treatment.
REFERENCES
1.Hirsh J., Dalen J., Guyatt G. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest. 2001. 119(S):1–2.
2.Laupacis A., Albers G., Dalen J., Dunn M., Feinberg W., Jacobson A. Antithrombotic therapy in atrial fibrillation. Chest. 1995. 108(4S):352–9.

3.Gullov AL., Koefoed BG., Petersen P. Bleeding complications to long-term oral anticoagulant therapy. J Thromb Thrombolysis. 1994. 1:17–25.
4.Landefeld CS., Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993. 95:315–28.

5.Levine MN., Raskob G., Landefeld S., Kearon C. Hemorrhagic complications of anticoagulant treatment. Chest. 2001. 119(S):108–21.

6.Bodin L., Horellou MH., Flaujac C., Loriot MA., Samama MM. A vitamin K epoxide reductase complex subunit-1 (VKORC1) mutation in a patient with vitamin K antagonist resistance. J Thromb Haemost. 2005. 3:1533–5.

7.D'Ambrosio RL., D'Andrea G., Cafolla A., Faillace F., Margaglione M. A new vitamin K epoxide reductase complex subunit-1 (VKORC1) mutation in a patient with decreased stability of CYP2C9 enzyme. J Thromb Haemost. 2007. 5:191–3.
8.Dericioglu N., Babaoglu MO., Saygi S., Bozkurt A., Yasar U. Warfarin resistance with poor CYP2C9 activity and CYP2C9∗1∗2 genotype. Ann Pharmacother. 2004. 38:899.

9.Harrington DJ., Underwood S., Morse C., Shearer MJ., Tuddenham EG., Mumford AD. Pharmacodynamic resistance to warfarin associated with a Val66Met substitution in vitamin K epoxide reductase complex subunit 1. Thromb Haemost. 2005. 93:23–6.

10.Gladman JR., Dolan G. Effect of age upon the induction and maintenance of anticoagulation with warfarin. Postgrad Med J. 1995. 71:153–5.

11.Loh PC., Morgan K., Wynne H. Anticoagulation of older patients: a need to modify current practice. Age Ageing. 2000. 29:551.

12.Gage BF., Eby C., Milligan PE., Banet GA., Duncan JR., McLeod HL. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost. 2004. 91:87–94.

13.The Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenousvein coronary-artery bypass grafts. N Engl J Med. 1997. 336:153–62.
14.Fang MC., Go AS., Hylek EM., Chang Y., Henault LE., Jensvold NG, et al. Age and the risk of warfarin-associated hemorrhage: the anti-coagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006. 54:1231–6.

15.Gan GG., Teh A., Goh KY., Chong HT., Pang KW. Racial background is a determinant factor in the maintenance dosage of warfarin. Int J Hematol. 2003. 78:84–6.

16.Kim MJ., Nafziger AN., Kashuba AD., Kirchheiner J., Bauer S., Gaedigk A, et al. Effects of fluvastatin and cigarette smoking on CYP2C9 activity measured using the probe S-warfarin. Eur J Clin Pharmacol. 2006. 62:431–6.

17.Lee VW., You JH., Lee KK., Chau TS., Waye MM., Cheng G. Factors affecting the maintenance stable warfarin dosage in Hong Kong Chinese patients. J Thromb Thrombolysis. 2005. 20:33–8.

18.Loebstein R., Yonath H., Peleg D., Almog S., Rotenberg M., Lubetsky A, et al. Interindividual variability in sensitivity to warfarin—Nature or nurture? Clin Pharmacol Ther. 2001. 70:159–64.

19.Kamali F., Khan TI., King BP., Frearson R., Kesteven P., Wood P, et al. Contribution of age, body size, and CYP2C9 genotype to anticoagulant response to warfarin. Clin Pharmacol Ther. 2004. 75:204–12.

20.Zhao F., Loke C., Rankin SC., Guo JY., Lee HS., Wu TS, et al. Novel CYP2C9 genetic variants in Asian subjects and their influence on maintenance warfarin dose. Clin Pharmacol Ther. 2004. 76:210–9.
21.Yu HC., Chan TY., Critchley JA., Woo KS. Factors determining the maintenance dose of warfarin in Chinese patients. Qjm. 1996. 89:127–35.

22.WHO. WHO expert committee on biological standardization. World Health Organ Tech Rep Ser. 1999. 889:i–vi. 1-111.
23.Burton ME, Shaw LM, editors. Applied pharmacokinetics & phar-macodynamics: Principles of therapeutic drug monitoring. 4th ed.Baltimore: Lippincott Williams & Wilkins;2006. p. 713–51.
24.Mozayani A., Raymon LP. .Handbook of drug interactions: A clinical and forensic guide. Totowa, NJ: Humana Press. 2004. 268–70.
25.Takahashi H., Wilkinson GR., Nutescu EA., Morita T., Ritchie MD., Scordo MG, et al. Different contributions of polymorphisms in VK-ORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics. 2006. 16:101–10.

26.Lombardi R., Chantarangkul V., Cattaneo M., Tripodi A. Measurement of warfarin in plasma by high performance liquid chromatography (HPLC) and its correlation with the international normalized ratio. Thromb Res. 2003. 111:281–4.

27.Osman A., Arbring K., Lindahl TL. A new high-performance liquid chromatographic method for determination of warfarin enantiomers. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. 826:75–80.

28.Sun S., Wang M., Su L., Li J., Li H., Gu D. Study on warfarin plasma concentration and its correlation with international normalized ratio. J Pharm Biomed Anal. 2006. 42:218–22.

29.Rettie AE., Wienkers LC., Gonzalez FJ., Trager WF., Korzekwa KR. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics. 1994. 4:39–42.

30.Rost S., Fregin A., Ivaskevicius V., Conzelmann E., Hortnagel K., Pelz HJ, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004. 427:537–41.

31.Krutzen E., Back SE., Nilsson-Ehle I., Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984. 104:955–61.
32.Rocco MV., Buckalew VM Jr., Moore LC., Shihabi ZK. Measurement of glomerular filtration rate using nonradioactive Iohexol: comparison of two one-compartment models. Am J Nephrol. 1996. 16:138–43.
33.Naidong W., Lee JW. Development and validation of a high-performance liquid chromatographic method for the quantitation of warfarin enantiomers in human plasma. J Pharm Biomed Anal. 1993. 11:785–92.

Fig. 1.
Chromatograms of 7-hydroxywarfarin (m/z 322.9 → 176.8), warfarin (m/z 307.0 → 160.9), and internal standard (chlorowarfarin; m/z 340.9 → 283.8).
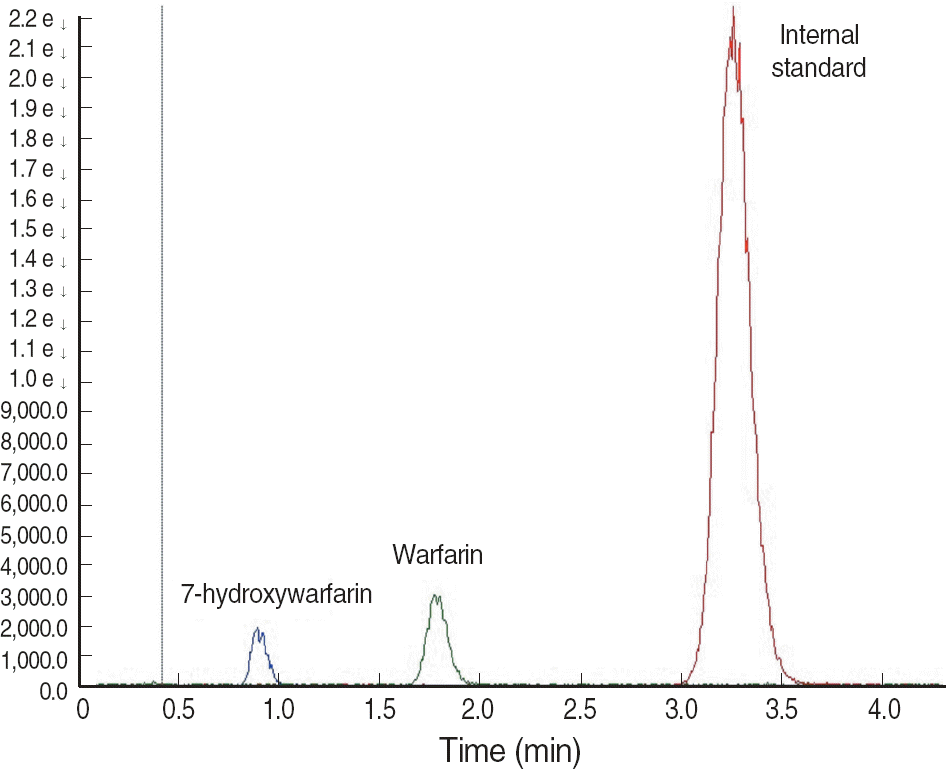
Fig. 2.
Correlation of PT INR, plasma warfarin concentration, and warfarin dose. Abbreviation: PT INR, prothrombin time international normalized ratio.
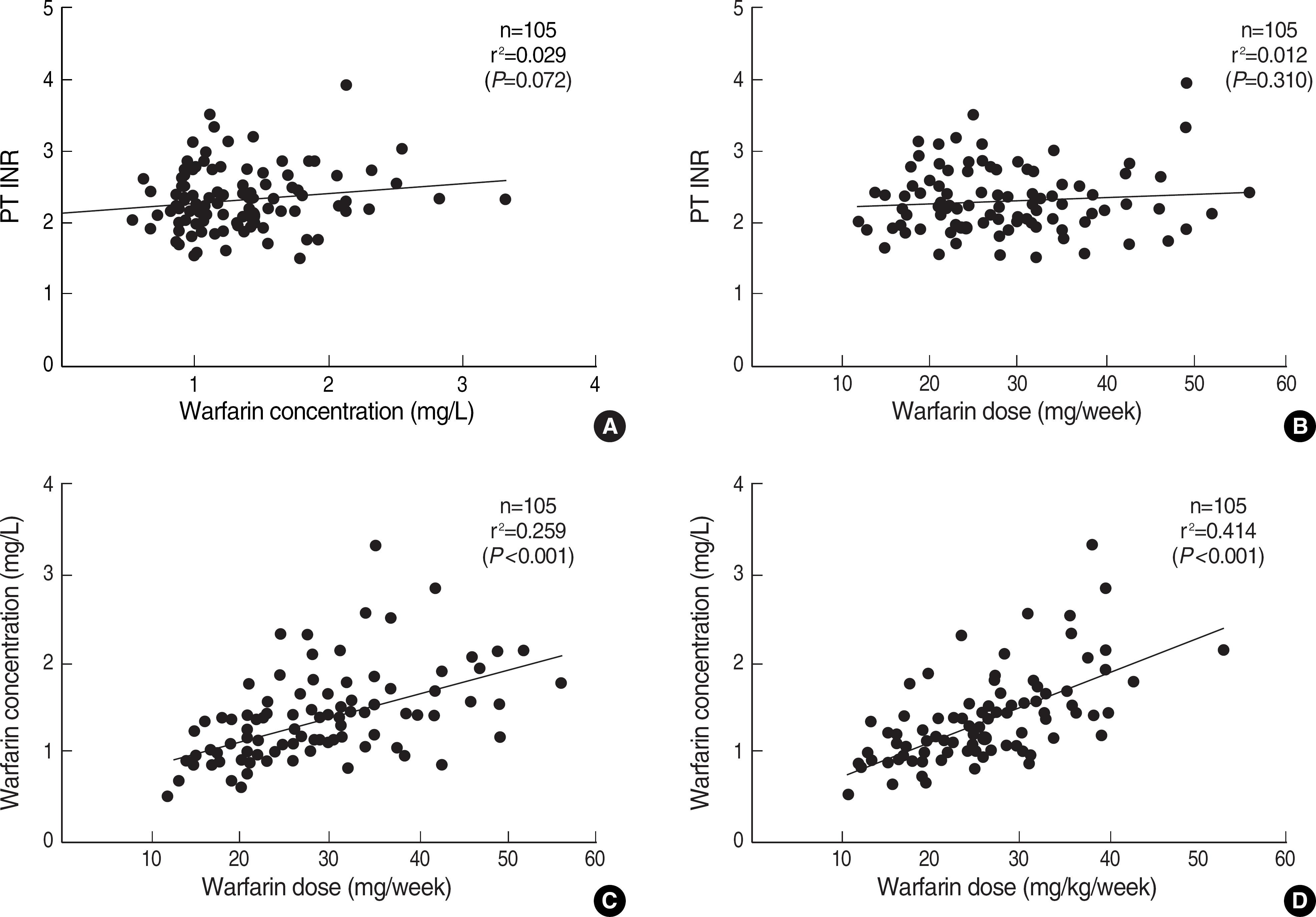
Fig. 3.
(A) The distributions of warfarin dose in 74 patients with PT INR within the target range of 2.0-3.0. (B) The total warfarin concentrations and the middle 95th percentile interval (0.6-2.6 mg/L) in this group. (C) The plasma warfarin to 7-hydroxywarfarin concentration ratio in this group. The vertical lines indicate the extent of normal distribution of the data.
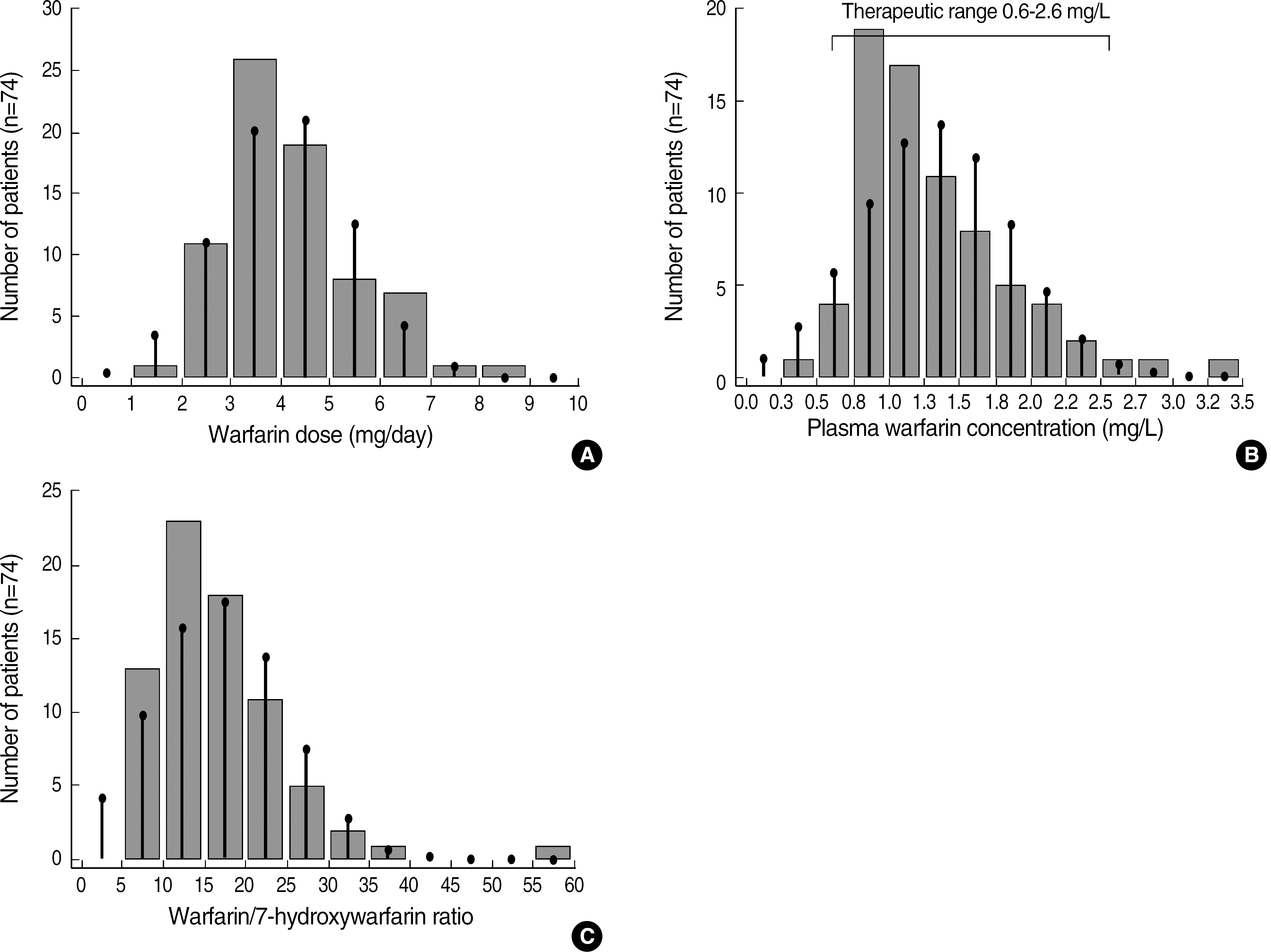
Table 1.
Characteristics of patients with atrial fibrillation (N=105)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download