Abstract
Background:
Majority of immune-mediated platelet refractoriness is caused by HLA alloimmunization and can be effectively managed by HLA-matched platelet transfusions. However, HLA class I-typed large-sized donor registry has not been well established in Korea. We evaluated the effectiveness of platelet transfusion using HLA crossmatch-compatible donors without HLA typing.
Methods:
Sixteen patients showing platelet refractoriness to random donor platelets (1 hr corrected count increment [CCI] <7,500/μL/m2) and HLA alloimmunization (class I panel reactive antibody >60%) were crossmatched with 78 platelet apheresis-eligible donors using National Institute of Health (NIH) and anti-human globulin (AHG) lymphocytotoxicity methods. NIH negative/AHG negative and NIH negative/AHG positive donors were selected as best and second choice donors, respectively.
Results:
Eleven patients (11/16, 69%) could find NIH-crossmatch negative donors and 27 donors (27/78, 35%) belonged to the best donors. To 8 patients, 32 apheresis platelet products from 19 donors were transfused. The mean 1 hr and 24 hr CCI values from the best donors were significantly higher than those from random donors (17,893 vs 2,358, P=0.003; 8,292 vs −614, P<0.001), whereas such differences were not observed for those from the second choice donors. Platelet storage time was inversely correlated with CCI values and platelets stored ≤10 hr after collection gave significantly higher CCI values. Neither ABO match nor donor status (related vs unrelated) affected the transfusion effectiveness.
REFERENCES
1.Klingemann HG., Self S., Banaji M., Deeg HJ., Doney K., Slichter SJ, et al. Refractoriness to random donor platelet transfusions in patients with aplastic anaemia: a multivariate analysis of data from 264 cases. Br J Haematol. 1987. 66:115–21.

2.Legler TJ., Fischer I., Dittmann J., Simson G., Lynen R., Humpe A, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997. 74:185–9.

3.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997. 337:1861–9.
4.Davis KB., Slichter SJ., Corash L. Corrected count increment and percentage platelet recovery as measures of posttransfusion platelet response: problems and a solution. Transfusion. 1999. 39:586–92.
5.Sacher RA., Kickler TS., Schiffer CA., Sherman LA., Bracey AW., Shulman IA. Management of patients refractory to platelet transfusion. Arch Pathol Lab Med. 2003. 127:409–14.

7.Kiefel V., König C., Kroll H., Santoso S. Platelet alloantibodies in transfused patients. Transfusion. 2001. 41:766–70.

8.Datema G., Stein S., Eijsink C., Mulder A., Claas FH., Doxiadis II. HLA-C expression on platelets: studies with an HLA-Cw1-specific human monoclonal antibody. Vox Sang. 2000. 79:108–11.

9.Moroff G., Garratty G., Heal JM., MacPherson BR., Stroncek D., Huang ST, et al. Selection of platelets for refractory patients by HLA matching and prospective crossmatching. Transfusion. 1992. 32:633–40.

10.Herzig RH., Terasaki PI., Trapani RJ., Herzig GP., Graw RG Jr. The relationship between donor-recipient lymphocytotoxicity and the transfusion response using HLA-matched platelet concentrates. Transfusion. 1977. 17:657–61.

11.Wu KK., Hoak JC., Koepke JA., Thompson JS. Selection of compatible platelet donors: a prospective evaluation of three cross-matching techniques. Transfusion. 1977. 17:638–43.

12.Park HD., Kim YH., Park YJ., Han KS., Park MH. An evaluation of HLA-matched platelet transfusion effect in patients with platelet refractoriness. Korean J Lab Med. 2004. 24:426–31. (박형두, 김양현, 박
윤준, 한규섭, 박명희. 혈소판불응증 환자의 HLA 적합혈소판 수혈효과.
대한진단검사의학회지 2004;24:426-31.).
13.Park MH., Han TH., Han KS., Ahn HS., Choi KH. Establishment of an HLA-matched platelet donor registry for the management of patients with platelet refractoriness. Korean J Blood Transfus. 1999. 10:203–14. (박명희, 한태희, 한규섭, 안효섭, 최경환. 혈소판수혈불응환
자의 치료를 위한 HLA 적합혈소판 헌혈자 등록제도의 수립. 대한수혈
학회지 1999;10:203-14.).
14.Vassallo RR Jr. New paradigms in the management of alloimmune refractoriness to platelet transfusions. Curr Opin Hematol. 2007. 14:655–63.

15.Bolgiano DC., Larson EB., Slichter SJ. A model to determine required pool size for HLA-typed community donor apheresis programs. Transfusion. 1989. 29:306–10.

16.Petz LD., Garratty G., Calhoun L., Clark BD., Terasaki PI., Gresens C, et al. Selecting donors of platelets for refractory patients on the basis of HLA antibody specificity. Transfusion. 2000. 40:1446–56.
17.McFarland JG. Matched apheresis platelets. McLeod BC, Price TH, editors. Apheresis: principles and practice. 2nd ed.Bethesda, MD: AABB press;2003. p. 199–220.
Fig. 1.
Distribution of 1 hr (A) and 24 hr CCI (B) values of platelet transfusions from NIH-crossmatch negative donors according to platelet storage time. Closed and open angles represent transfusions from NIH-/AHG- and NIH-/AHG+ donors, respectively. Horizontal lines in A and B represent CCI values of 7,500 and 5,000, respectively.
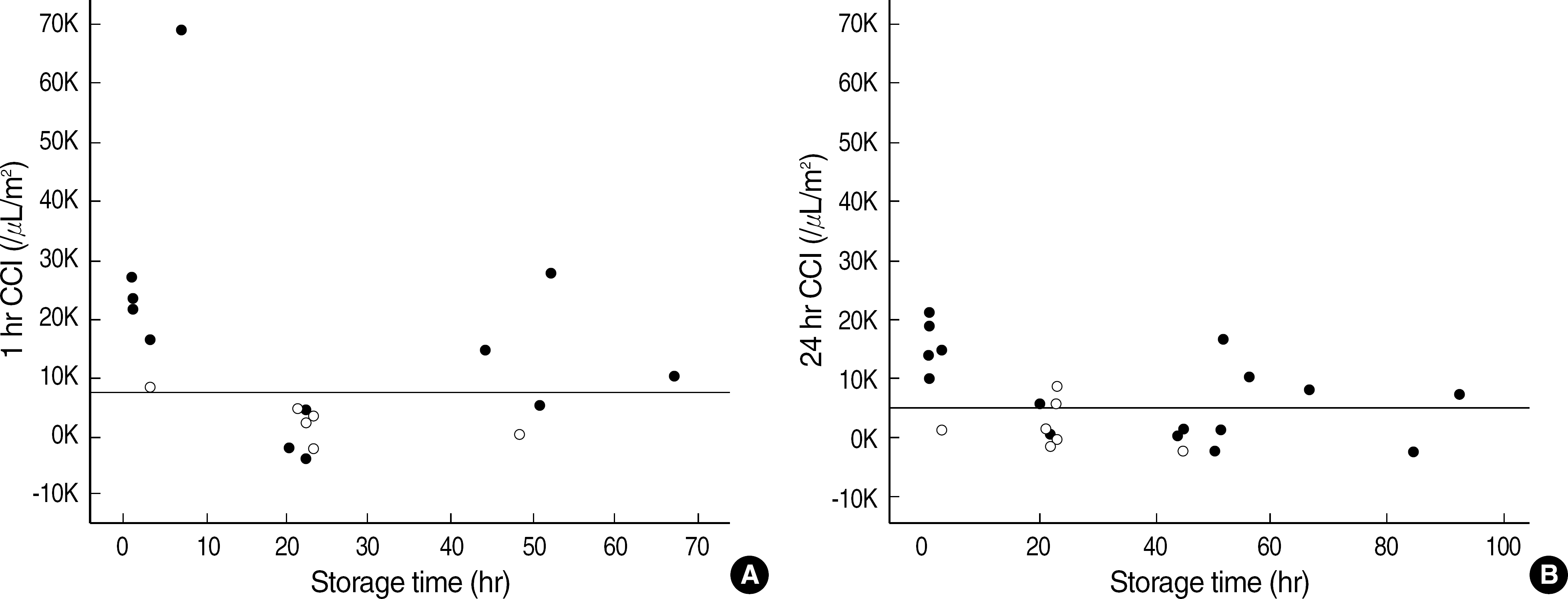
Fig. 2.
Inverse correlation between platelet storage time and 1 hr (A) and 24 hr CCI (B) values of platelet transfusions from NIH-/AHG- donors.
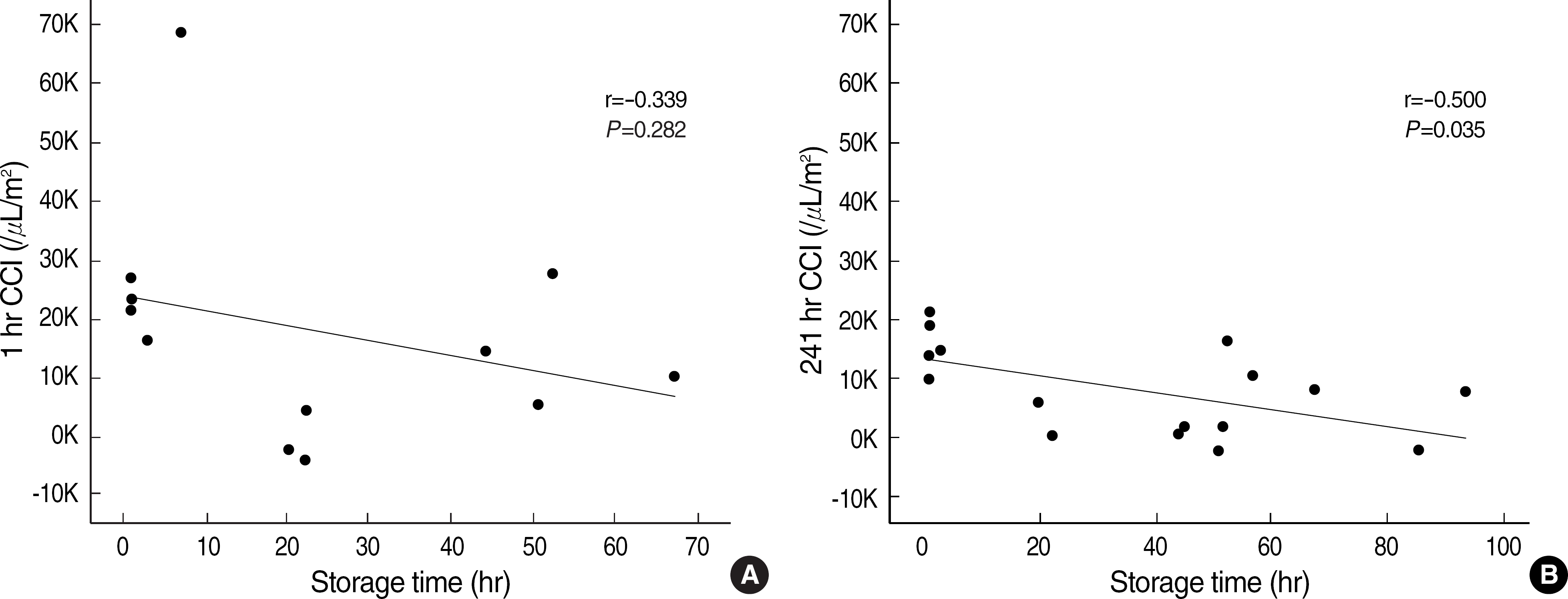
Table 1.
ELISA-PRA and HLA crossmatch results in patients with platelet refractoriness
| Patient No. | PRA | HLA crossmatch results | ||||
|---|---|---|---|---|---|---|
| PRA % | OD max∗ | OD >0.7 (%)† | NIH-/AHG- | NIH-/AHG+ | NIH- | |
| 1-1‡ | 100 | 2.281 | 100 | 0% (0/4) | 0% (0/4) | 0% (0/4) |
| 2 | 100 | 2.280 | 100 | 0% (0/2) | 0% (0/2) | 0% (0/2) |
| 3 | 100 | 1.441 | 100 | 0% (0/7) | 0% (0/7) | 0% (0/7) |
| 4 | 100 | 1.959 | 98 | 0% (0/1) | 0% (0/1) | 0% (0/1) |
| 5 | 100 | 1.360 | 90 | 0% (0/1) | 0% (0/1) | 0% (0/1) |
| 6 | 100 | 1.305 | 85 | 40% (2/5) | 0% (0/5) | 40% (2/5) |
| 7 | 98 | 2.209 | 73 | 0% (0/5) | 40% (2/5) | 40% (2/5) |
| 8 | 95 | 1.197 | 70 | 100% (1/1) | 0% (0/1) | 100% (1/1) |
| 9 | 95 | 1.683 | 68 | 67% (2/3) | 33% (1/3) | 100% (3/3) |
| 10 | 100 | 2.069 | 38 | 35% (12/34) | 32% (11/34) | 68% (23/34) |
| 11 | 100 | 1.198 | 38 | 0% (0/1) | 100% (1/1) | 100% (1/1) |
| 12 | 100 | 1.547 | 30 | 100% (3/3) | 0% (0/3) | 100% (3/3) |
| 13 | 98 | 1.213 | 28 | 0% (0/2) | 0% (0/2) | 0% (0/2) |
| 14 | 63 | 1.314 | 10 | 100% (3/3) | 0% (0/3) | 100% (3/3) |
| 15 | 100 | 0.714 | 3 | 100% (2/2) | 0% (0/2) | 100% (2/2) |
| 1-2‡ | 90 | 0.308 | 0 | 50% (1/2) | 50% (1/2) | 100% (2/2) |
| 16§ | 100 | NA | NA | 50% (1/2) | 0% (0/2) | 50% (1/2) |
| Total | 35% (27/78) | 21% (16/78) | 55% (43/78) | |||
Table 2.
Effectiveness of platelet transfusions in 8 patients with platelet refractoriness according to HLA crossmatch results
| Platelet transfusions (N) | 1 hr CCI (/μL/m2) | 24 hr CCI (/μL/m2) | ||||
|---|---|---|---|---|---|---|
| Mean±SD (N) | P∗ | ≥7,500 | Mean±SD (N) | P∗ | ≥5,000 | |
| Random donors (39) | 2,358±4,517 (28) | 4 (14%) | -614±1,248 (15) | 0 (0%) | ||
| NIH-XM- donors (32) | 12,895±17,297 (18) | 0.009 | 9 (50%)† | 6,526±7,653 (25) | 0.001 | 13 (52%)‡ |
| NIH-/AHG- (24) | 17,893±19,356 (12) | 0.003 | 8 (67%)§ | 8,292±8,067 (18) | <0.001 | 11 (61%)‡ |
| NIH-/AHG+ (8) | 2,901±3,624 (6) | 0.470 | 1 (17%) | 1,983±4,059 (7) | 0.148 | 2 (29%) |
Table 3.
Effectiveness of platelet transfusions from NIH-crossmatch negative donors according to platelet storage time
| Platelet transfusions (N) | 1 hr CCI (/μL/m2) | 24 hr CCI (/μL/m2) | ||||
|---|---|---|---|---|---|---|
| Mean±SD (N) | P | ≥7,500 | Mean±SD (N) | P | ≥5,000 | |
| NIH-XM- donors | ||||||
| ≤10 hr (8) | 27,737±21,289 (6) | 0.007∗ | 6 (100%)‡ | 14,609±7,050 (7) | 0.002∗ | 6 (86%) |
| >10 hr (24) | 5,475±8,726 (12) | 3 (25%) | 3,382±5,270 (18) | 7 (39%) | ||
| ≤24 hr (19) | 13,367±19,665 (13) | 0.588† | 6 (46%) | 8,256±8,129 (15) | 0.174† | 9 (60%) |
| >24 hr (13) | 11,668±10,430 (5) | 3 (60%) | 3,931±6,397 (10) | 4 (40%) | ||
| NIH-/AHG- | ||||||
| ≤10 hr (7) | 31,581±21,346 (5) | 0.028∗ | 5 (100%) | 16,774±4,505 (6) | 0.003∗ | 6 (100%)§ |
| >10 hr (17) | 8,115±10,744 (7) | 3 (43%) | 4,052±5,700 (12) | 5 (42%) | ||
| ≤24 hr (13) | 19,597±23,222 (8) | 1.000† | 5 (63%) | 11,971±8,189 (9) | 0.077† | 7 (78%) |
| >24 hr (11) | 14,483±9,603 (4) | 3 (75%) | 4,614±6,386 (9) | 4 (44%) | ||
| NIH-/AHG+ | ||||||
| ≤10 hr (1) | 8,513 (1) | 0.143∗ | 1 (100%) | 1,621 (1) | 0.617∗ | 0 (0%) |
| >10 hr (7) | 1,778±2,639 (5) | 0 (0%) | 2,044±4,443 (6) | 2 (33%) | ||
| ≤24 hr (6) | 3,399±3,815 (5) | 0.380† | 1 (20%) | 2,683±3,956 (6) | 0.134† | 2 (33%) |
| >24 hr (2) | 409 (1) | 0 (0%) | -2,217 (1) | 0 (0%) | ||
Table 4.
Effectiveness of platelet transfusions from NIH- and AHG-crossmatch negative donors according to the ABO match status
| Platelet transfusions (N) | 1 hr CCI (/μL/m2) | 24 hr CCI (/μL/m2) | ||||
|---|---|---|---|---|---|---|
| Mean±SD (N) | P∗ | ≥7,500 | Mean±SD (N) | P∗ | ≥5,000 | |
| ABO identical (11) | 19,713±28,963 (5) | 0.570 | 2 (40%) | 3,982±4,935 (7) | 0.135 | 3 (43%) |
| ABO non-identical (13) | 16,592±11,086 (7) | 6 (86%) | 11,035±8,645 (11) | 8 (73%) | ||
Table 5.
Effectiveness of platelet transfusions from NIH-crossmatch negative donors according to the donor status (related vs unrelated)
| Platelet transfusions (N) | 1 hr CCI (/μL/m2) | 24 hr CCI (/μL/m2) | ||||
|---|---|---|---|---|---|---|
| Mean±SD (N) | P∗ | ≥7,500 | Mean±SD (N) | P∗ | ≥5,000 | |
| NIH-XM- donors | ||||||
| Related (15) | 16,338±20,192 (10) | 0.534 | 6 (60%) | 8,251±9,755 (11) | 0.584 | 5 (45%) |
| Unrelated (17) | 8,592±10,793 (8) | 3 (38%) | 5,171±5,518 (14) | 8 (57%) | ||
| NIH-/AHG- | ||||||
| Related (11) | 24,554±24,310 (6) | 0.337 | 5 (83%) | 11,141±9,998 (8) | 0.328 | 5 (63%) |
| Unrelated (13) | 11,231±11,260 (6) | 3 (50%) | 6,014±5,673 (10) | 6 (60%) | ||
| NIH-/AHG+ | ||||||
| Related (4) | 4,014±3,489 (4) | 0.355 | 1 (25%) | 544±1,838 (3) | 0.724 | 0 (0%) |
| Unrelated (4) | 674±3,777 (2) | 0 (0%) | 3,063±5,203 (4) | 2 (50%) | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download