Abstract
Pallister-Killian syndrome (PKS) is a rare disorder characterized cytogenetically by tetrasomy 12p for isochromosome of the short arm of chromosome 12. PKS is diagnosed by prenatal genetic analysis through chorionic villous sampling, genetic amniocentesis, and cordocentesis, or by chromosomal analysis of skin fibroblasts, but is not usually detected by chromosomal analysis of peripheral blood cells. Herein, we report a case of a gravida at 23 weeks gestation with pulmonary stenosis and right ventricular dilation of the heart which were detected by sonography. Fluorescence in situ hybridization and a multicolor banding technique were performed to verify the diagnosis as 47,XX, +mar.ish i(12)(p10)(TEL++)[16]/46,XX[4], and an autopsy confirmed the cardiac anomalies detected on antenatal sonography.
Go to : 
REFERENCES
1.Pallister PD., Meinsner LF., Elajalde BR., Franche U., Herrmann J., Spranger J, et al. The Pallister mosaic syndrome. Birth Defects. 1977. 12:103–10.
2.Killian W., Teschler-Nicola M. Case report 72: mental retardation, unusual facial appearance, abnormal hair. Synd Ident. 1981. 7:6–7.
3.Langford K., Hodgson S., Seller M., Maxwell D. Pallister-Killian syndrome presenting through nuchal translucency screening for trisomy 21. Prenat Diagn. 2000. 20:670–2.

4.Gilgenkrantz S., Droulle P., Schweitzer M., Foliguet B., Chadefaux B., Lombard M. Mosaic tetrasomy 12p. Clin Genet. 1985. 28:495–502.

5.Chiesa J., Hoffet M., Rousseau O. Pallister-Killian syndrome [i(12p)]: first pre-natal diagnosis using cordocentesis in the second trimester confirmed by in situ hybridization. Clin Genet. 1998. 54:294–302.

6.Schaefer GB., Jochar R., Muneer R., Sangar WG. Clinical variability of tetrasomy12p. Clin Genet. 1997. 51:102–8.
7.Doray B., Girard-Lemaire F., Gasser B., Baldauf JJ., de Gitter B., Spizzo M, et al. Pallister-Killian syndrome: difficulties of prenatal diagnosis. Prenat Diagn. 2002. 22:470–7.

8.Vermeesch JR., Melotte C., Salden I., Riegel M., Trifnov V., Polityko A, et al. Tetrasomy 12pter-12p13.31 in a girl with partial Pallister-Killian syndrome phenotype. Eur J Med Genet. 2005. 48:319–27.

10.Han JY., Kim TG., Shaffer LG. Detection of the Pallister-Killian syndrome by G-banding and FISH in cultured skin fibroblasts. Korean J Clin Pathol. 1998. 18:284–7. (한진영, 김태겸, Shaffer LG. 배양된 피부 섬유아세포에서 G-banding 과 FISH 로 진단한 Pallister-Killian 증후 군. 대한임상병리학회지 1998;18:284-7.).
11.Lee JS., Kim SH., Choi JA., Nam SY., Kim SY. A case of Pallister-Killian syndrome. J Korean Pediatr Soc. 2000. 43:274–7. (이주석, 김성훈,최정아, 남상욱, 김수영. Pallister-Killian syndrome 1례. 소아과 2000;43:274-7.).
12.Kim MH., Park SY., Kim MY., Lee BY., Lee MH., Ryu HM. Prenatal diagnosis of Pallister-Killian syndrome in two fetuses with increased nuchal translucency. Prenat Diagn. 2008. 28:454–6.

13.Chudoba I., Plesch A., Lörch T., Lemke J., Claussen U., Senger G. High resolution multicolor-banding: a new technique for refined FISH analysis of human chromosomes. Cytogenet Cell Genet. 1999. 84:156–60.

14.Senger G., Ragoussis J., Trowsdale J., Sheer D. Fine mapping of human MHC class II region within chromosome 6p21 and evaluation of probe ordering using fluorescence in situ hybridization. Cytogenet Cell Genet. 1993. 64:49–53.
15.Wenger SL., Steele MW., Yu WD. Risk effect of maternal age in Pallister i(12p) syndrome. Clin Genet. 1998. 34:181–4.

16.Wilson RD., Harrison K., Clarke LA., Yong SL. Tetrasomy 12p (Pallister-Killian syndrome): ultrasound indicators and confirmation by interphase fish. Prenat Diagn. 1994. 14:787–92.

17.Turleau C., Simon-Bouy B., Austruy E., Grisard MC., Lemaire F., Molina-Gomes D, et al. Parental origin and mechanisms of formation of three cases of 12p tetrasomy. Clin Genet. 1996. 50:41–6.
18.Lalatta F., Salmona S., Fogliani R., Rizzuti T., Nicolini U. Prenatal diagnosis of genetic syndromes may be facilitated by serendipitous findings at fetal blood sampling. Prenat Diagn. 1998. 18:834–7.

19.Hoffman JI., Christianson R. Congenital heart disease in a cohort of 19,502 births with long term follow up. Am J Cardiol. 1978. 42:641–7.
20.Wimalasundera RC., Gardiner HM. Congenital heart disease and aneuploidy. Prenat Diagn. 2004. 24:1116–22.

21.Polityko AD., Goncharova E., Shamgina L., Drozdovskaja N., Podleschuk L., Abramchik E, et al. Pallister-Killian syndrome: rapid decrease of isochromosome 12p frequency during amniocyte subculturing. Conclusion for strategy of prenatal cytogenetic diagnostics. J Histochem Cytochem. 2005. 53:361–4.

Go to : 
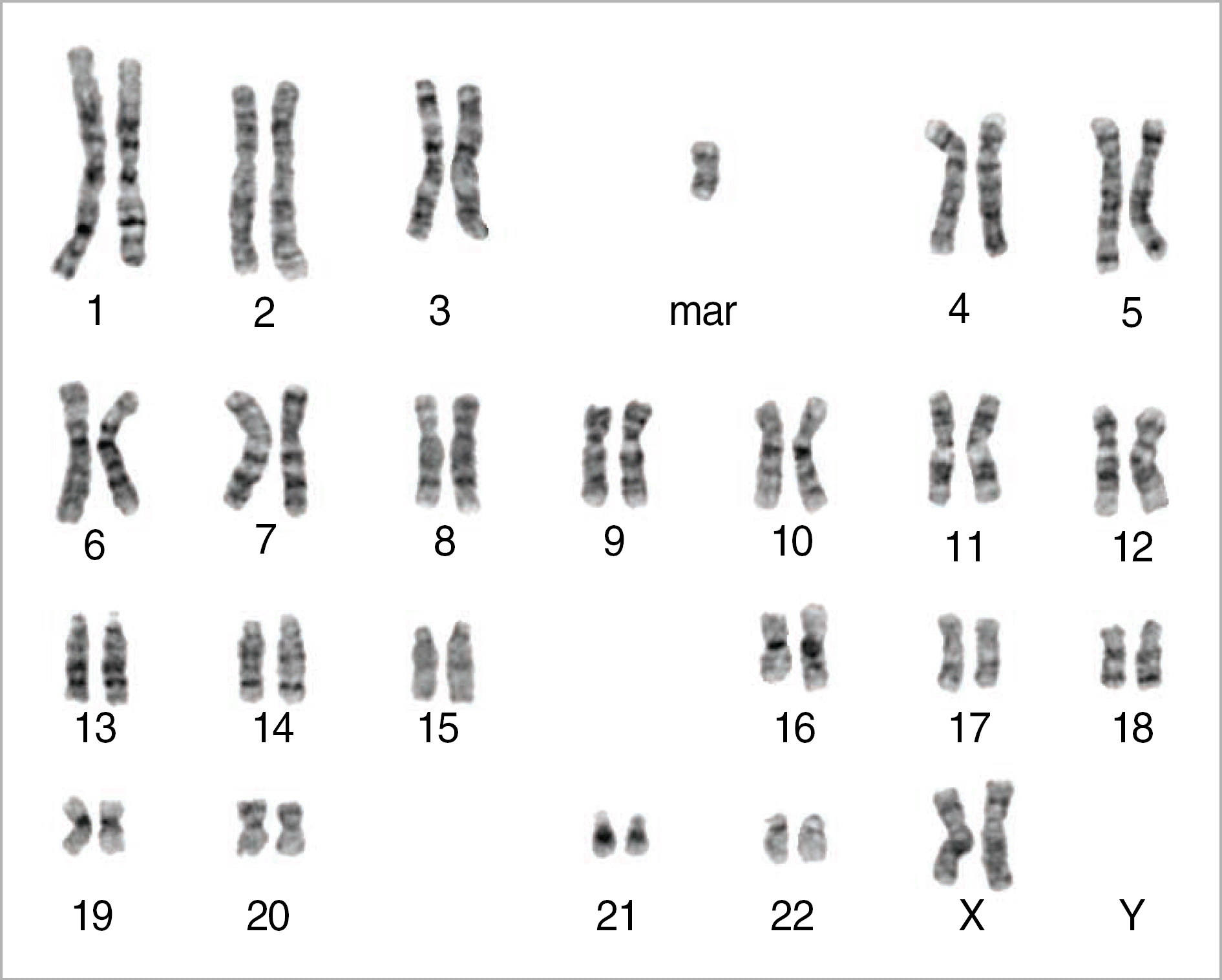 | Fig. 1.Conventional cytogenetic analysis on GTG-banded chromosomes from amniotic fluid of the fetus showed 47,XX,+mar in 16 cells and 46,XX in 4 cells. |
 | Fig. 2.Fluorescence in situ hybridization (FISH) analysis using a TEL/AML1 (LSI TEL/AML1 Dual Color, Extra Signal Fusion Translocation Probe, Abbot Molecular/Vysis, Des Plaines, IL, USA) probe showed two green (TEL) signals located symmetrically on both arms of the marker chromosome (arrows). |
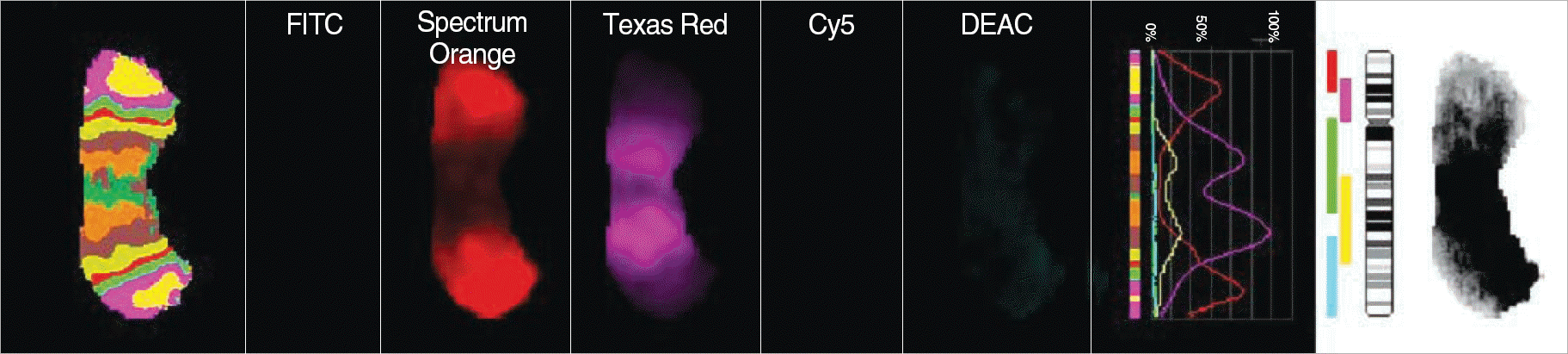 | Fig. 3.The chromosome 12-specific mBAND probe kit, XCyte 12 (MetaSystems, Altlussheim, Germany), was hybridized to metaphase cells. Each fluorochrome was detected by adequate filter and the signals were analyzed by Isis/mFISH imaging software (MetaSystems). This analysis refined chromosome regions involved in isochromosome 12. The multiple band pattern confirmed that i(12) contained all regions of 12p. |
Table 1.
Prenatally diagnosed Pallister-Killian syndromes presenting with cardiac defects
| Reference | Cardiac defects | Extracardiac abnormalities | G-wks | Degree of mosaicism | Molecular cytogenetic method |
|---|---|---|---|---|---|
| Gilgenkrantz et al. [4] | Ebstein's anomaly | Polyhydroamnios, rhizomelic micromelia, edema (neck, trunk), facial dysmorphia with macrocephaly∗, Club feet∗, sacral dimple∗ | 26 | 100% of amniocytes | - |
| Wilson et al. [17] | Tetralogy of Fallot | Polyhydroamnios, diaphragmatic hermia, micromelia, facial dysmorphia, pleural effusion, anal stenosis∗, rectovaginal fistula∗ | 26 | 16/18 amniocytes | FISH |
| Turleau et al. [18] | Right hypertrophy, abnormal tricuspid valve | Polyhydroamnios, micromelia, craniofacial abnormalities∗, clinodactyly∗, hypoplastic lung, sacrococcygeal pit∗ | 30 | 100% of amniocytes | - |
| Lalatta et al. [19] | Atrio-ventricular septal defect | Diaphragmatic hernia, macrocephaly, facial abnormalites∗, abscent testicles∗ | 19 | 0% of 20 clones of amniocytes | - |
| Langford et al. [3] | Hypoplastic left heart | Increased nuchal translucency, hydrops, diaphragmatic hernia∗, micromelia, postaxial polydactyly∗, talipes∗ camptodactyly,∗ imperforate anus∗ | 15 | 6% of Chorionic villi | ISH |
| Doray et al. [7] | Tetralogy of fallot | Dandy walker malformatrion, rhizomelic micromelia, diaphragmatic hernia, club hand, craniofacial dysmorphism∗, flexed toes∗, various deformity∗, adrenal hypertrophy∗ | 16 | 9/100 Cord blood Lymphocytes | - |
| Abad et al. [16] | Right ventricular hypertrophy, VSD, severe TR | Increased nuchal translucency, facial abnormalities∗ | 13 | 100% of amniocytes | |
| Present case | Right ventricular dilatation, PS | Dolicocephaly, low set ear∗ | 23 | 16/20 of amniocytes | FISH, mBAND |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download