Abstract
Background:
We investigated the characteristics of the mononuclear cells remaining in the leuko-reduction system (LRS) chambers of Trima Accel® in comparison with those of standard buffy coat cells, and evaluated their potential for differentiation into dendritic cells.
Methods:
Twenty-six LRS chambers of Trima Accel® were collected after platelet pheresis from healthy adults. Flow cytometric analysis for T, B, NK, and CD14+ cells was performed and the number of CD34+ cells was counted. Differentiation and maturation into dendritic cells were induced using CD14+ cells seperated via Magnetic cell sorting (MACS®) Seperation (Miltenyi Biotec Inc., USA).
Results:
Total white blood cell (WBC) count in LRS chambers was 10.8× 108 (range 7.7-18.0× 108). The median values (range) of proportions of each cells were CD4+ T cell 29.6% (18.7-37.6), CD8+ T cell 27.7% (19.2-40.0), B cell 5.5% (2.2-12.1), NK cell 15.7% (13.7-19.9), and CD14+ cells 12.4% (8.6-32.3) respectively. Although total WBC count was significantly higher in the buffy coat (whole blood of 400 mL) than the LRS chambers, the numbers of lymphocytes and monocytes were not statistically different. The numbers of B cells and CD4+ cells were significantly higher in the buffy coat than the LRS chambers (P<0.05). The median value (range) of CD34+ cells obtained from the LRS chambers was 0.9 × 106 (0.2-2.6 × 106). After 7 days of cytokine-supplemented culture, the CD14+ cells were successfully differentiated into dendritic cells.
REFERENCES
1.Shehata N., Lin Y., Pendergrast J., Branch DR. Cellular therapies: a Canadian blood services research and development symposium. Transfus Med Rev. 2007. 21:317–36.

2.Mellman I., Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001. 106:255–8.
3.Sniecinski I., O'Donnell MR., Nowicki B., Hill LR. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988. 71:1402–7.

4.Meyer TP., Zehnter I., Hofmann B., Zaisserer J., Burkhart J., Rapp S, et al. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005. 307:150–66.

5.Ebner S., Neyer S., Hofer S., Nussbaumer W., Romani N., Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001. 252:93–104.

6.Adams MR., Dumont LJ., McCall M., Heaton WA. Clinical trial and local process evaluation of an apheresis system for preparation of white cell-reduced platelet components. Transfusion. 1998. 38:966–74.

7.Neron S., Thibault L., Dussault N., Cote G., Ducas E., Pineault N, et al. Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: a new source of viable human blood cells. Transfusion. 2007. 47:1042–9.

8.Dietz AB., Bulur PA., Emery RL., Winters JL., Epps DE., Zubair AC, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006. 46:2083–9.

9.Karimi K., Boudreau JE., Fraser K., Liu H., Delanghe J., Gauldie J, et al. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther. 2007. 16:411–8.
10.Lee JJ., Nam CE., Nam JH., Lee HC., Chung IJ., Park MS, et al. Generation of cytotoxic donor CD8+ T cells against relapsing leukemic cells following allogeneic transplantation by stimulation with leukemic cell- or leukemic lysate pulsed donor cell-derived dendritic cells. Leuk Res. 2004. 28:517–24.

11.Weitkamp JH., Crowe JE Jr. Blood donor leukocyte reduction filters as a source of human B lymphocytes. Biotechniques. 2001. 31:464–6.

12.Teleron AA., Carlson B., Young PP. Blood donor white blood cell reduction filters as a source of human peripheral blood-derived endothelial progenitor cells. Transfusion. 2005. 45:21–5.

13.Ivanovic Z., Duchez P., Morgan DA., Hermitte F., Lafarge X., Chevaleyre J, et al. Whole-blood leuko-depletion filters as a source of CD 34+ progenitors potentially usable in cell therapy. Transfusion. 2006. 46:118–25.
14.Pickl WF., Majdic O., Kohl P., Stockl J., Riedl E., Scheinecker C, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996. 157:3850–9.
Fig. 1.
Wright-Giemsa stain (×400) of cells isolated from LRS chambers (A), CD14+ cells seperated by MACS® Seperation (B), immature dendritic cells on Day 5 of culture (C), and mature dendritic cells on Day 7 of culture (D).
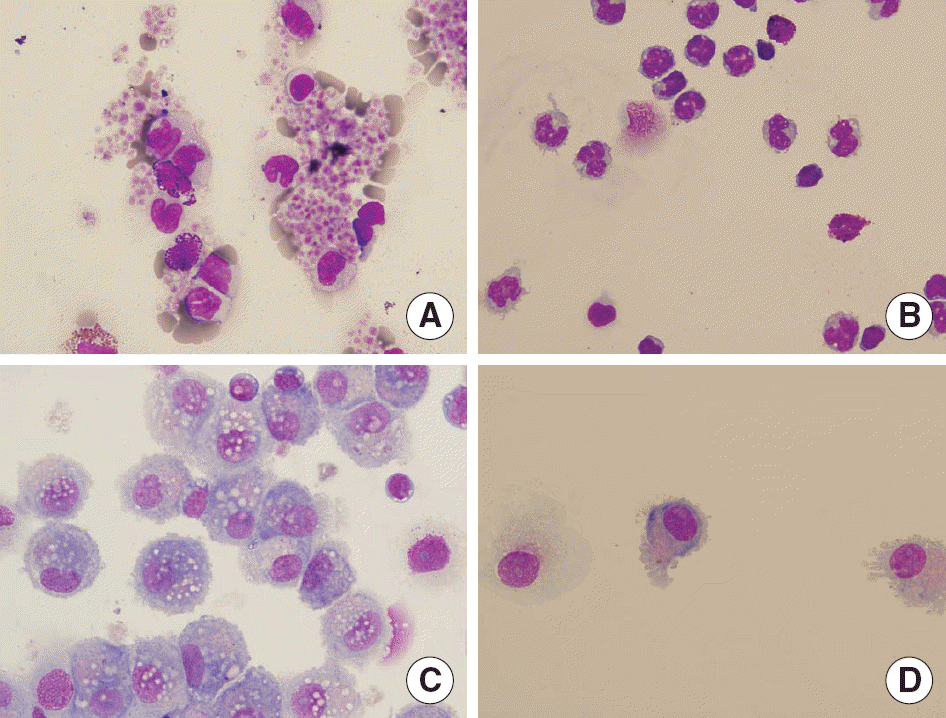
Fig. 2.
The immunophenotypic characteristics of mature dendritic cells (mDCs) and immature dendritic cells (iDCs) on Day 0, 5 and 7 of culture. Phenotypic analysis was performed with CD14+ cells separated from LRS chamber (A) and buffy coat (D) on Day 0; iDCs generated by culturing CD14+ cells from LRS (B) and buffy coat (E) with GM-CSF and IL-4 for 5 days; and mDCs generated from LRS CD14+ cell-derived iDCs (C) and buffy coat-CD14+cell-derived iDC (F) for additional 48 hr in the presence of TNF- α, IL-1β, IL-6 and PGE2.
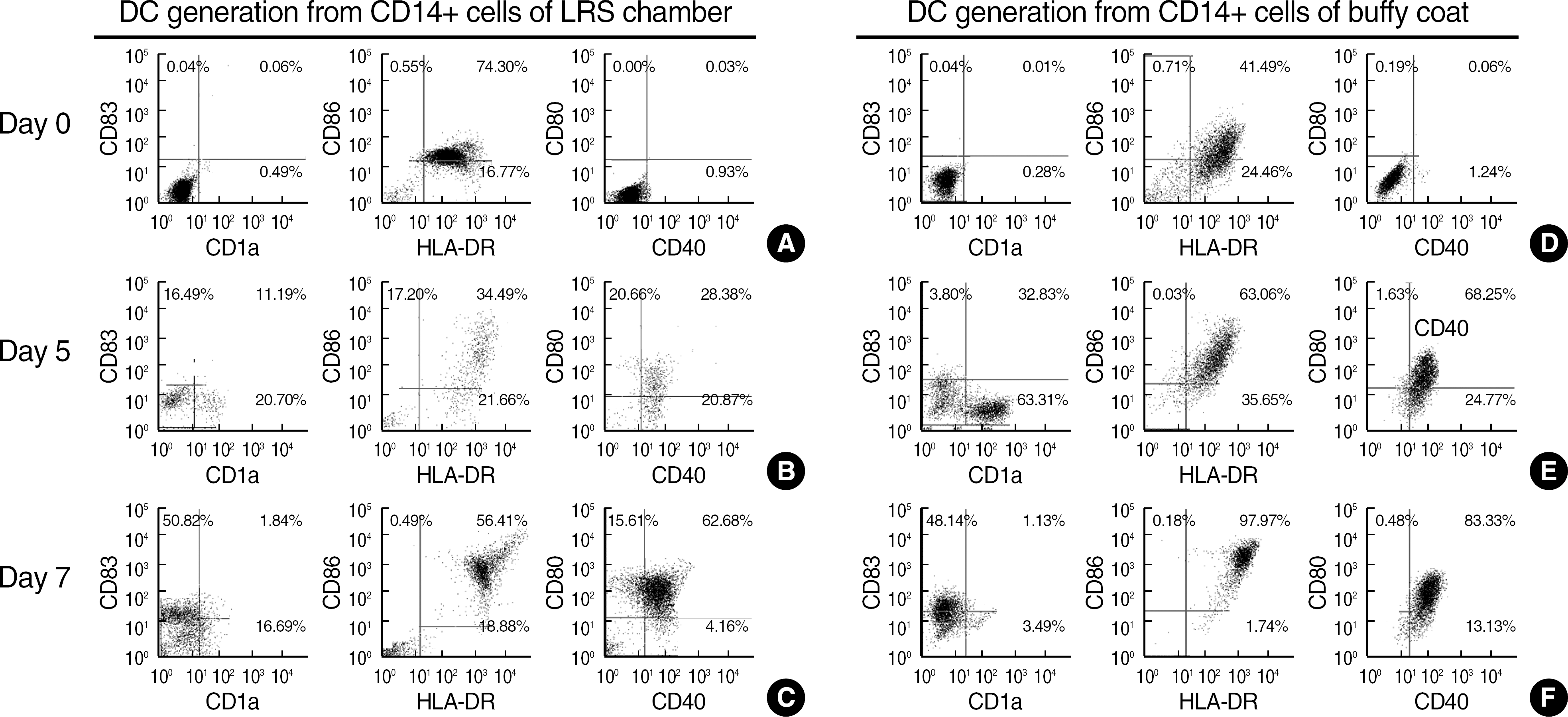
Fig. 3.
Mature dendritic cells (mDCs) generated from CD14+ monocytes possess strong allostimulatory capacitites. Allostimulatory capacities of DCs from LRS-derived MNCs (A) and DCs from buffy coat-derived MNCs (B) were analyzed by using identical allogeneic T cells. Allogeneic T cells proliferated more vigorously when they were stimulated with mDCs than stimulated with immature DCs (iDCs) on various DC:T cell ratio. Results shown are mean values (circle)±SD (bar) of triplicate wells.
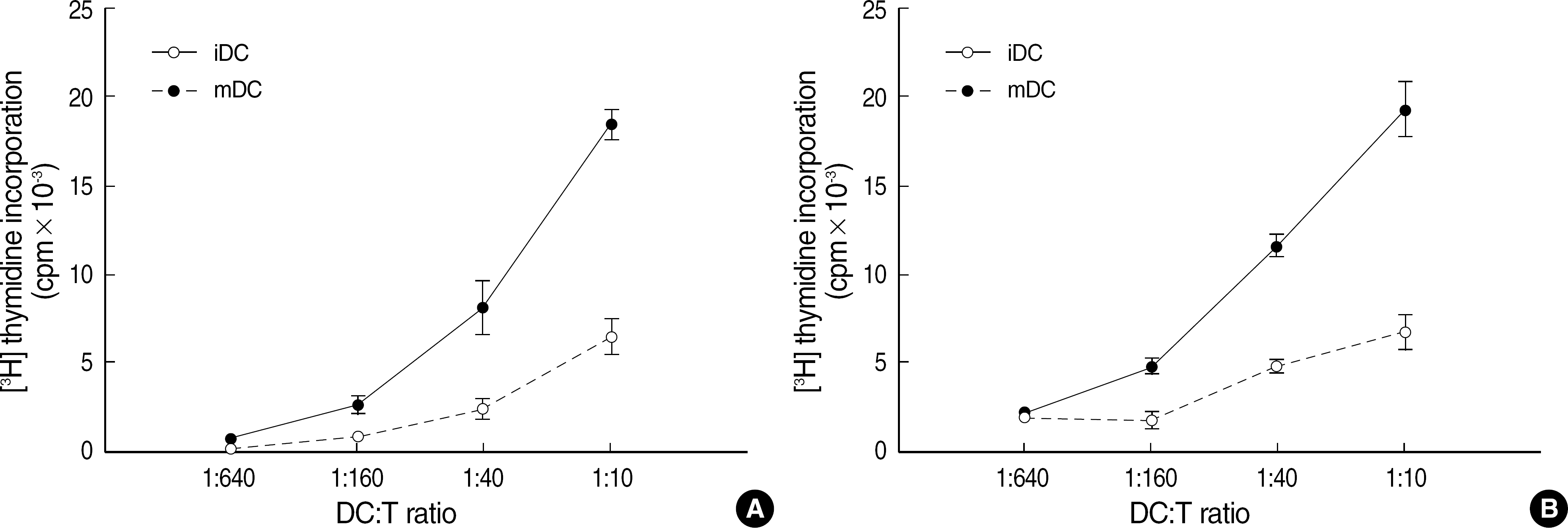
Table 1.
Total WBC count and differential count: comparision between cells isolated from LRS chambers and buffy coats seperated from whole blood
Table 2.
Flow cytometric characterization of mononuclear cells: comparison between LRS chamber-derived and buffy coat-derived cells
| Component | LRS chamber (n=18) | Buffy coat (n=9) | P value∗ | ||
|---|---|---|---|---|---|
| Median (%) | Range (%) | Median (%) | Range (%) | ||
| CD4+ cell | 29.6 | 18.7-37.6 | 38.5 | 26.0-51.9 | 0.016 |
| CD8+ cell | 27.7 | 19.2-40.0 | 25.2 | 14.2-33.1 | 0.177 |
| B cell | 5.5 | 2.2-12.1 | 10.2 | 3.4-16.2 | 0.016 |
| NK cell | 15.7 | 13.7-19.9 | 12.9 | 4.8-26.5 | 0.272 |
| CD14+ cell | 12.4 | 8.6-32.3 | 8.7 | 3.2-15.2 | 0.062 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download