Abstract
Background:
Many immunochromatography (ICA) kits for anti-human immunodeficiency virus type (HIV) antibody (Ab) have been introduced to improve the accessibility of HIV Ab tests. However, qualified evaluation reports for HIV rapid tests are not enough to validate their performances. Meta-analysis for the performances of the HIV Ab rapid tests was performed in this study.
Methods:
PubMed database was searched with combination of search terms, ‘human immunodeficiency virus’, ‘HIV Ab’, ‘rapid test’, ‘immunochromatography’, ‘performance’, ‘sensitivity’, and ‘specificity’. Criteria of inclusion were performance studies for HIV ICA kits with serum or EDTA whole blood. Methodological qualities were evaluated with standards for reporting of diagnostic accuracy studies (STARD) checklists by two investigators. Homogeneity among selected studies was evaluated and then pooled sensitivity and specificity were calculated. Positive and negative predictive values were simulated with presumed HIV prevalence in Korea.
Results:
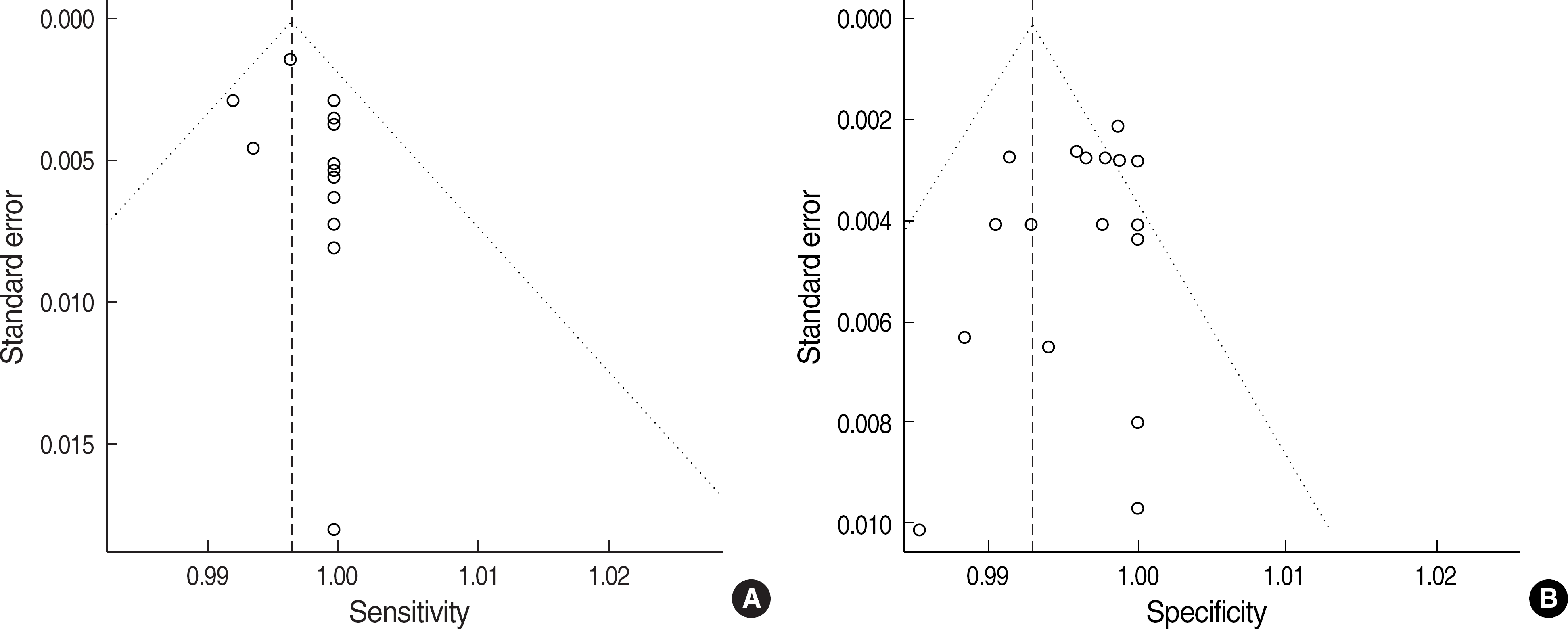
Twenty-three studies were selected from 12 high-qualified papers with STARD checklists. The performance of 23 studies were found to be heterogeneous (P<0.1) and random effect model was used. Pooled sensitivity was 99.71% (95% CI: 99.45-99.97%) and pooled specificity was 99.27% (95% CI: 98.83-99.70%). With HIV prevalence of 0.03%, positive and negative predictive values were presumed to be 3.936% and 99.999%, respectively.
Conclusions:
This meta-analysis for HIV ICA rapid tests showed good performance. In consideration of low positive predictive values of HIV rapid tests, confirmation by enzyme immunoassay or Western blot is still needed. This study would be helpful in evaluating and establishing proper performance guideline for those kits not fully evaluated.
REFERENCES
1.Centers for Disease Control (CDC). Public Health Service guidelines for counseling and antibody testing to prevent HIV infection and AIDS. Morb Mortal Wkly Rep. 1987. 36:509–15.
2.Behets F., Bishagara K., Disasi A., Likin S., Ryder RW., Brown C, et al. Diagnosis of HIV infection with instrument-free assays as an alternative to the ELISA and western blot testing strategy: an evaluation in Central Africa. J Acquir Immune Defic Syndr. 1992. 5:878–82.
3.Bhore AV., Sastry J., Patke D., Gupte N., Bulakh PM., Lele S, et al. Sensitivity and specificity of rapid HIV testing of pregnant women in India. Int J STD AIDS. 2003. 14:37–41.

4.Brattegaard K., Kouadio J., Adom ML., Doorly R., George JR., De Cock KM. Rapid and simple screening and supplemental testing for HIV-1 and HIV-2 infections in west Africa. AIDS. 1993. 7:883–5.

5.Downing RG., Otten RA., Marum E., Biryahwaho B., Alwano-Edyegu MG., Sempala SD, et al. Optimizing the delivery of HIV counseling and testing services: the Uganda experience using rapid HIV antibody test algorithms. J Acquir Immune Defic Syndr Hum Retrovirol. 1998. 18:384–8.

6.Foglia G., Royster GD 4th., Wasunna KM., Kibaya R., Malia JA., Calero EK, et al. Use of rapid and conventional testing technologies for human immunodeficiency virus type 1 serologic screening in a rural Kenyan reference laboratory. J Clin Microbiol. 2004. 42:3850–2.

7.Centers for Disease Control and Prevention (CDC). Advancing HIV prevention: new strategies for a changing epide—United States, 2003. Morb Mortal Wkly Rep. 2003. 52:329–32.
8.Greenwald JL. Routine rapid HIV testing in hospitals: another opportunity for hospitalists to improve care. J Hosp Med. 2006. 1:106–12.

9.Bossuyt PM., Reitsma JB., Bruns DE., Gatsonis CA., Glasziou PP., Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003. 138:40–4.

10.Kim S., Oh HB., Cha CH., Choi SE., An HY., Lee KJ. Quality evaluation of the performance study of diagnostic tests using STARD checklist and Meta-Analysis for the pooled sensitivity and specificity of third generation anti-HCV EIA tests. Korean J Lab Med. 2006. 26:307–15. (김솔잎, 오흥범, 차충환, 최성은, 안홍엽, 이관제. STARD 를 이용한진단검사법성능평가연구의질평가와메타분석을통한 3 세대 HCV 효소면역 항체검사의 통합민감도와 특이도 분석. 대한진단검사의학회지 2006;26:307-15.).
11.Hwang SH., Oh HB., Choi SE., Kim HH., Chang CL., Lee EY, et al. Meta-analysis for the pooled sensitivity and specificity of hepatitis B surface antigen rapid tests. Korean J Lab Med. 2008. 28:160–8. (황상현, 오흥범, 최성은, 김형회, 장철훈, 이은엽 등. 메타분석을 이용한 B 형 간염항원신속검사법의통합민감도와통합특이도분석. 대한진단검사의학회지 2008;28:160-8.).

12.Lien TX., Tien NT., Chanpong GF., Cuc CT., Yen VT., Soderquist R, et al. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2000. 62:301–9.

13.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. 315:629–34.

14.Macaskill P., Walter SD., Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001. 20:641–54.

15.Peters JL., Sutton AJ., Jones DR., Abrams KR., Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006. 295:676–80.

16.Irwig L., Macaskill P., Glasziou P., Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995. 48:119–30.

17.Wang JS., Kee MK., Suh SD., Shin HS., Kim HS., Kim SS. Post-evaluation of rapid HIV kits in the Korean market by an anti-HIV EQAS panel. J Virol Methods. 2007. 141:141–5.

18.Yang BM., Choi WJ. Economic impect of HIV/AIDS in Korea. [Research Report]. http://www.aidsinfo.or.kr/Board/Down_Files/7th_Symposium_02.pdf. (양봉민 및 최운정. 한국에서 HIV/AIDS 감염의 경제적 영향. [연구보고서]. http://www.aidsinfo.or.kr/Board/Down. Files/7th Symposium 02.pdf.).
19.Menard D., Mairo A., Mandeng MJ., Doyemet P., Koyazegbe T., Rochigneux C, et al. Evaluation of rapid HIV testing strategies in under equipped laboratories in the Central African Republic. J Virol Methods. 2005. 126:75–80.
20.Menard D., Mavolomade EE., Mandeng MJ., Talarmin A. Advantages of an alternative strategy based on consecutive HIV serological tests for detection of HIV antibodies in Central African Republic. J Virol Methods. 2003. 111:129–34.
21.Rouet F., Ekouevi DK., Inwoley A., Chaix ML., Burgard M., Bequet L, et al. Field evaluation of a rapid human immunodeficiency virus (HIV) serial serologic testing algorithm for diagnosis and differentiation of HIV type 1 (HIV-1), HIV-2, and dual HIV-1-HIV-2 infections in West African pregnant women. J Clin Microbiol. 2004. 42:4147–53.

22.Urassa W., Nozohoor S., Jaffer S., Karama K., Mhalu F., Biberfeld G. Evaluation of an alternative confirmatory strategy for the diagnosis of HIV infection in Dar Es Salaam, Tanzania, based on simple rapid assays. J Virol Methods. 2002. 100:115–20.

23.World Health Organization. HIV assays: operational characteristics. Report 14. Simple/rapid tests. 2004.
24.World Health Organization. HIV assays: operational characteristics. Report 15. Antigen/Antibody ELISAs. 2004.
25.Vijayakumar TS., David S., Selvaraj K., Viswanathan T., Kannangai R., Sridharan G. Performance of a rapid immunochromatographic screening test for detection of antibodies to human immunodeficiency virus type 1 (HIV-1) and HIV-2: experience at a tertiary care hospital in South India. J Clin Microbiol. 2005. 43:4194–6.

26.Wesolowski LG., MacKellar DA., Facente SN., Dowling T., Ethridge SF., Zhu JH, et al. Post-marketing surveillance of OraQuick whole blood and oral fluid rapid HIV testing. AIDS. 2006. 20:1661–6.

27.Delaney KP., Branson BM., Uniyal A., Kerndt PR., Keenan PA., Jafa K, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006. 20:1655–60.

28.Aidoo S., Ampofo WK., Brandful JA., Nuvor SV., Ansah JK., Nii-Trebi N, et al. Suitability of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus in Ghana, West Africa. J Clin Microbiol. 2001. 39:2572–5.

29.Arai H., Petchclai B., Khupulsup K., Kurimura T., Takeda K. Evaluation of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus. J Clin Microbiol. 1999. 37:367–70.

30.Burgess-Cassler A., Barriga Angulo G., Wade SE., Castillo Torres P., Schramm W. A field test for the detection of antibodies to human immunodeficiency virus types 1 and 2 in serum or plasma. Clin Diagn Lab Immunol. 1996. 3:480–2.

31.Ferreira Junior OC., Ferreira C., Riedel M., Widolin MR., Barbosa-Junior A. Evaluation of rapid tests for anti-HIV detection in Brazil. AIDS. 2005. 19(S4):70–5.
32.Ketema F., Zeh C., Edelman DC., Saville R., Constantine NT. Assessment of the performance of a rapid, lateral flow assay for the detection of antibodies to HIV. J Acquir Immune Defic Syndr. 2001. 27:63–70.

Fig. 2.
Estimates of sensitivity and specificity of HIV Ab rapid tests. Points indicate estimates of sensitivities (A) and specificities (B). Vertical lines are 95% confidence intervals for estimates.
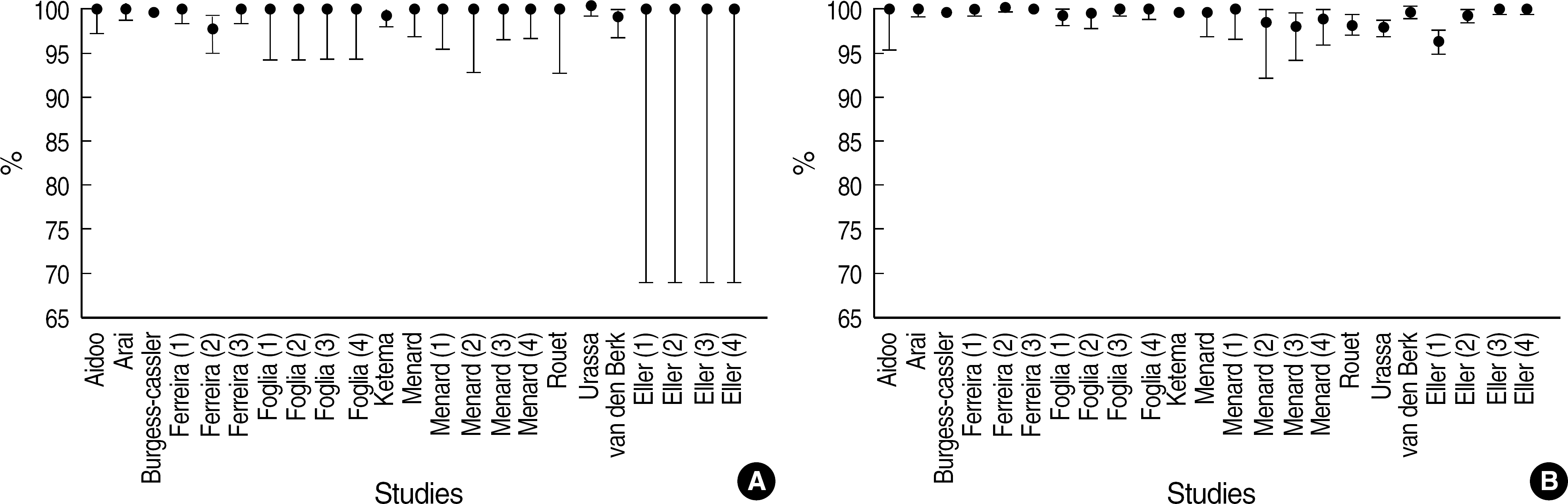
Table 1.
Literature survey on both sensitivity and specificity of HIV Ab rapid test kits using serum or whole blood
Abbreviations: TP, true positive; FN, false negative; FP, false positive; TN, true negative; Sn, sensitivity; Sp, specificity; PA, particle agglutination assay; WB, Western blot assay; EIA, enzyme immunoassay; RQ-PCR, real-time quantitative polymerase chain reaction; MEIA, microparticle enzyme immunoassay.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download