Abstract
Background:
Near-infrared light (NIR, 0.8-1.5 μm light) has been used in therapeutic devices for various injuries such as infected, ischemic and hypoxic wound. NIR-emitting technology has been developed recently in Korea. We hypothesized that NIR may have an anti-inflammatory effect and investigated the effect of NIR-irradiated media on cell culture.
Methods:
Three kinds of cell lines, CAPE (vascular endothelial cell), NIH3T3 (fibroblast), and RD (smooth muscle cell) cells were cultured for 4 days in 10% FBS-containing media (1×104 cells/well), which were irradiated or not irradiated (control) by Eco-NFIR Drive (Model #0210, Ecowavetech, Korea). The cells were stimulated by 10 mcg/mL of bacterial lipopolysaccharide (LPS) or sodium nitroprusside (SNP). Cellular proliferation was measured by methylthiazol tetrazolium assay. Expression of interleukin (IL)-1 beta and nitric oxide was measured by ELISA. Expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) was measured by immunofluorescence staining.
Results:
NIR-irradiated medium was favorable for CAPE cell proliferation (N=8, P=0.000). IL-1 beta secretion from LPS-stimulated NIH3T3 cells incubated in the NIR medium was below that of control medium (N=4, P=0.026). Nitrate production seemed to be low in NIR-irradiated medium although statistically insignificant (N=4, P=0.076). Expression of iNOS of the LPS-stimulated cells was decreased in NIR medium, however, Cox-2 expression was not different between the two media.
REFERENCES
1.Wong-Riley MT., Liang HL., Eells JT., Chance B., Henry MM., Buchmann E, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005. 280:4761–71.
2.Eells JT., Wong-Riley MT., VerHoeve J., Henry M., Buchman EV., Kane MP, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004. 4:559–67.

3.Danno K., Mori N., Toda K., Kobayashi T., Utani A. Near-infrared irradiation stimulates cutaneous wound repair: laboratory experiments on possible mechanisms. Photodermatol Photoimmunol Photomed. 2001. 17:261–5.
4.Kandolf-Sekulovic L., Kataranovski M., Pavlovic MD. Immunomodulatory effects of low-intensity near-infrared laser irradiation on contact hypersensitivity reaction. Photodermatol Photoimmunol Photomed. 2003. 19:203–12.

5.Mochizuki-Oda N., Kataoka Y., Cui Y., Yamada H., Heya M., Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett. 2002. 323:207–10.

6.Oh WS., In JY., Ha KH., Hong KH. Application of polarized light irradiation to meralgia paresthetica. Korean J Anesthesiol. 2000. 38:183–6. (오완수, 인준용, 하경호, 홍기혁. 직선편광 근적외선 조사에 의한 지각이상성대퇴신경통의치료경험. 대한마취과학회지 2000;38:183-6.).

7.Han SM., Lee SC. The change of blood flow velocity of radial artery after linear polarized infrared light radiation near the stellate ganglion: comparing with the stellate ganglion block. Korean J Pain. 2001. 14:37–40. (한승문 및 이상철. 성상신경절 부위의 직선편광 근적외선조사후요골동맥에서의혈류속도의변화: 성상신경절차단술과의비
교. 대한통증학회지 2001;14:37-40.).
8.Kim J., Otzel D., Kim W., Janelle CM. Near-infrared light and expectancy effects on maximal isokinetic strength performance: a randomized, double-blind, placebo-controlled study. J Strength Cond Res. 2006. 20:378–82.

9.Choi CH., Lee HS., Seo HS., Kim SG., Shin IH. Biochemical changes and recovery after half-course marathon. J Korean Orthop Soc Sports Med. 2008. 7:45–9. (최창혁, 이현섭, 서헌석, 김상경, 신임희. 하프코스마라톤후체내의생화학적변화및회복. 대한정형외과스포츠의학회지 2008;7:45-9.).
10.Kim SS., Kim WJ., Kim HE. The study of thermo-physiological responses with near infrared lighted garment at a hot environment. J Korean Soc Cloth Ind. 2005. 7:665–72. (김성숙, 김우종, 김희은. 서열환경에서 근적외선 조사의복 착용시의 온열생리반응. 한국의류산업학회지 2005; 7:665–72.).
11.Park KH., Park YM., Seul KJ., Ghim SY. The indirect effects of the near infra-red light-treated materials on microbial growth. Korean J Microbiol Biotechnol. 2005. 33:222–5. (박경화, 박유미, 설경조, 김사열. 근적외선을 처리한 생활용품의 항균 효과. 한국미생물생명공학회지 2005;33:222-5.).
12.Song JS. Review of tumor necrosis factor inhibitors on rheumatoid arthritis. J Korean Rheum Assoc. 2007. 14:1–14. (송정수. 류마티스관절염 치료에서 종양괴사인자억제제 사용에 대한 고찰. 대한류마티스학회지 2007;14:1-14.).

13.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Method. 1986. 89:271–7.
14.Kim SG., Choe JY., Choi CH., Choi GH., Kim JK. Effect of culture condition on chondrocyte viability isolated from articular cartilage. Korean J Lab Med. 2004. 24:237–43. ((김상경, 최정윤, 최창혁, 최기환, 김종기. 관절연골조직으로부터 연골세포 일차배양시 배양방법에 따른 세포의생존력. 대한진단검사의학회지 2004;24:237-43.).
15.Kim S., Temple MM., Chen AC., Sah RL. Effect of nitric oxide on cartilage homeostasis and chondrocyte viability. J Orhto Res. 2001. ;S18.
16.Komorowska M., Cuissot A., Czarnoleski A., Bialas W. Erythrocyte response to near-infrared radiation. J Photochem Photobiol B. 2002. 68:93–100.

17.Whelan HT., Connelly JF., Hodgson BD., Barbeau L., Post AC., Bullard G, et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002. 20:319–24.

18.Whelan HT., Smits RL Jr., Buchman EV., Whelan NT., Turner SG., Margolis DA, et al. Effect of NASA light-emitting diodes irradiation on wound healing. J Clin Laser Med Surg. 2001. 19:305–14.
Fig. 1.
Methylthiazol tetrazolium test result of the cells. NIR-irradiated medium provided favorable condition for CAPE cells in terms of cellular mitochondrial enzyme (succinate dehydrogenase) actvity (mean±SE, N=8, P=0.000). However, there was no significant difference of cellular proliferation of NIH3T3 cell (mean±SE, N=16, P=0.170) and RD cell (mean±SE, N=8, P=0.295) between NIR-irradiated medium (N, –Δ–) and control medium (C, –○–).
Abbreviations: C, control medium; N, NIR-irradiated medium; MTT, methylthiazol tetrazolium; NIR, near-infrared irradiated.
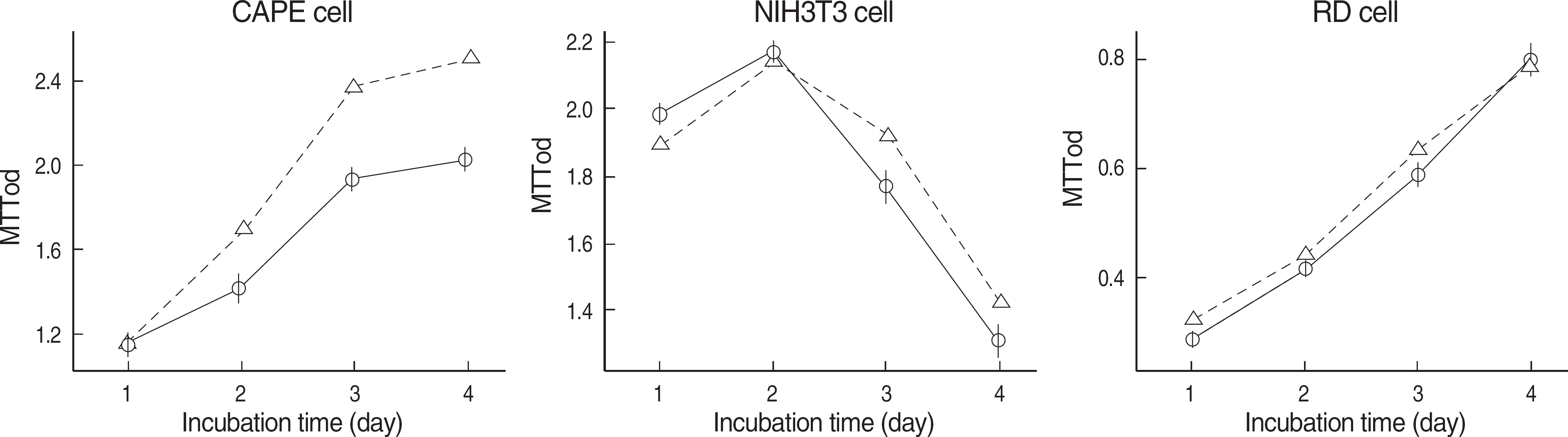
Fig. 2.
Methylthiazol tetrazolium test result of SNP-stimulated NIH3T3 cells. The SNP-stimulated NIH3T3 cells incubated in the NIR-irradiated medium (–Δ–) proliferated more than the cells incubated in the control medium (–○–) at day 2 (mean±SE, N=16, P=0.000). Abbreviations: See Fig. 1.
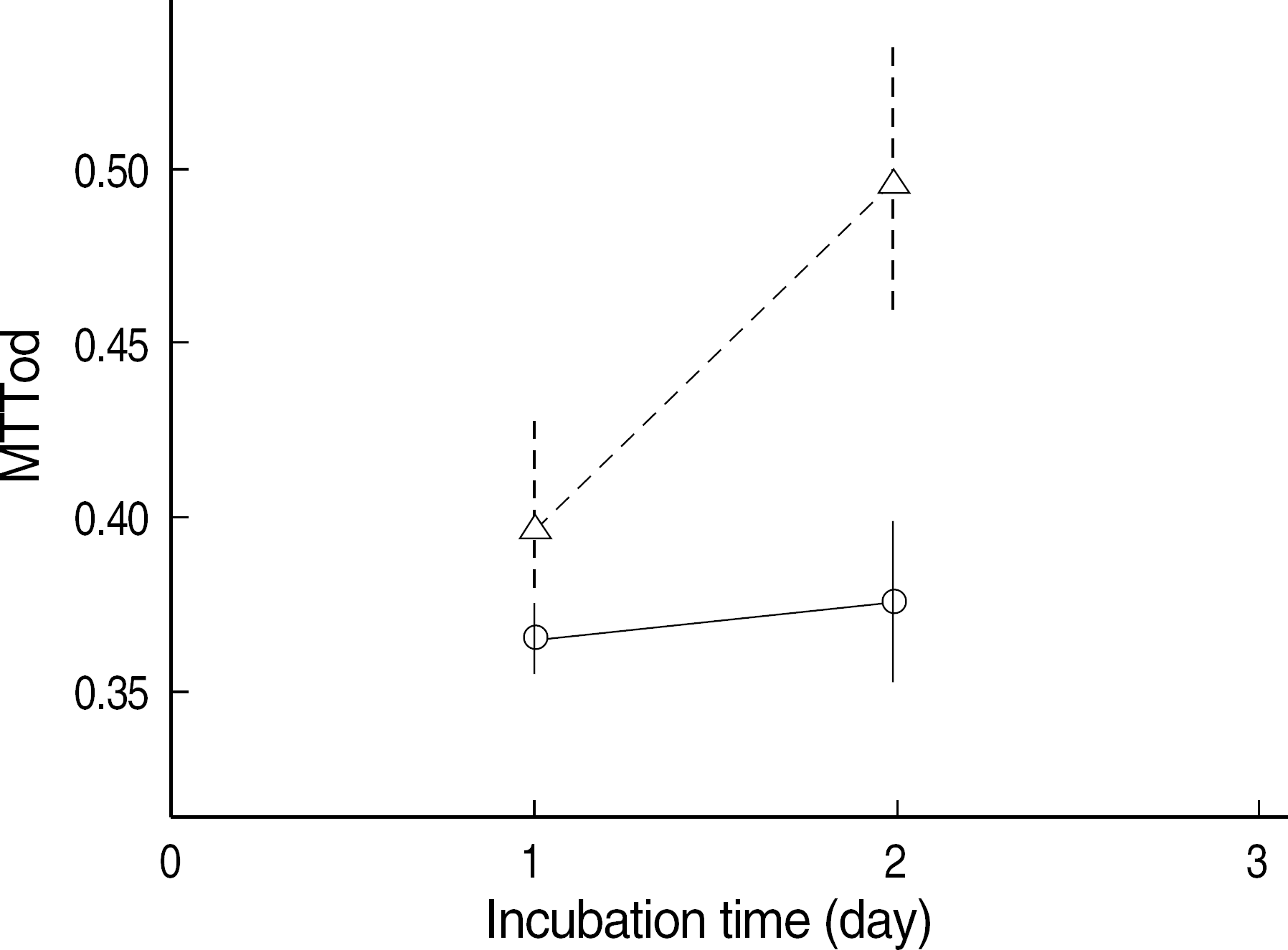
Fig. 3.
IL-1 beta concentration of the supernatant of NIH3T3 cells which were stimulated with LPS (10 mcg/mL). The IL-1 beta secretion of NIH3T3 cells incubated in NIR-irradiated medium (–Δ–) was below that in the control medium (–○–) (mean±SE, N=4, P=0.026).
Abbreviations: See Fig. 1.
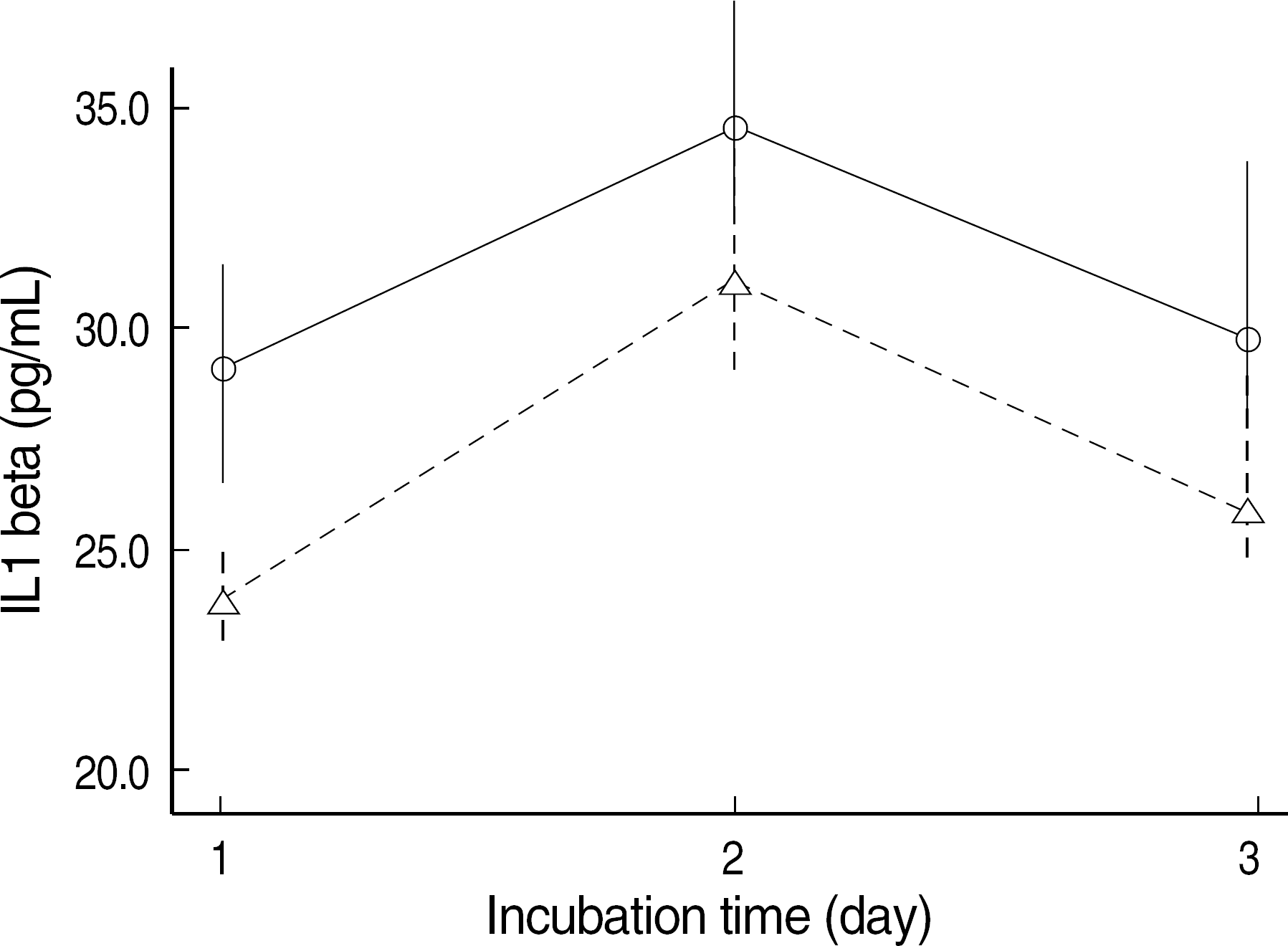
Fig. 4.
Nitric oxide production of NIH3T3 cells which were stimulated with LPS (10 mcg/mL). The NO level of NIH3T3 cells incubated in NIR-irradiated medium (NIR) was lower than that in the control medium (control), although statistically insignificant (mean±SE, N=4, P=0.076).
Abbreviations: See Fig. 1.
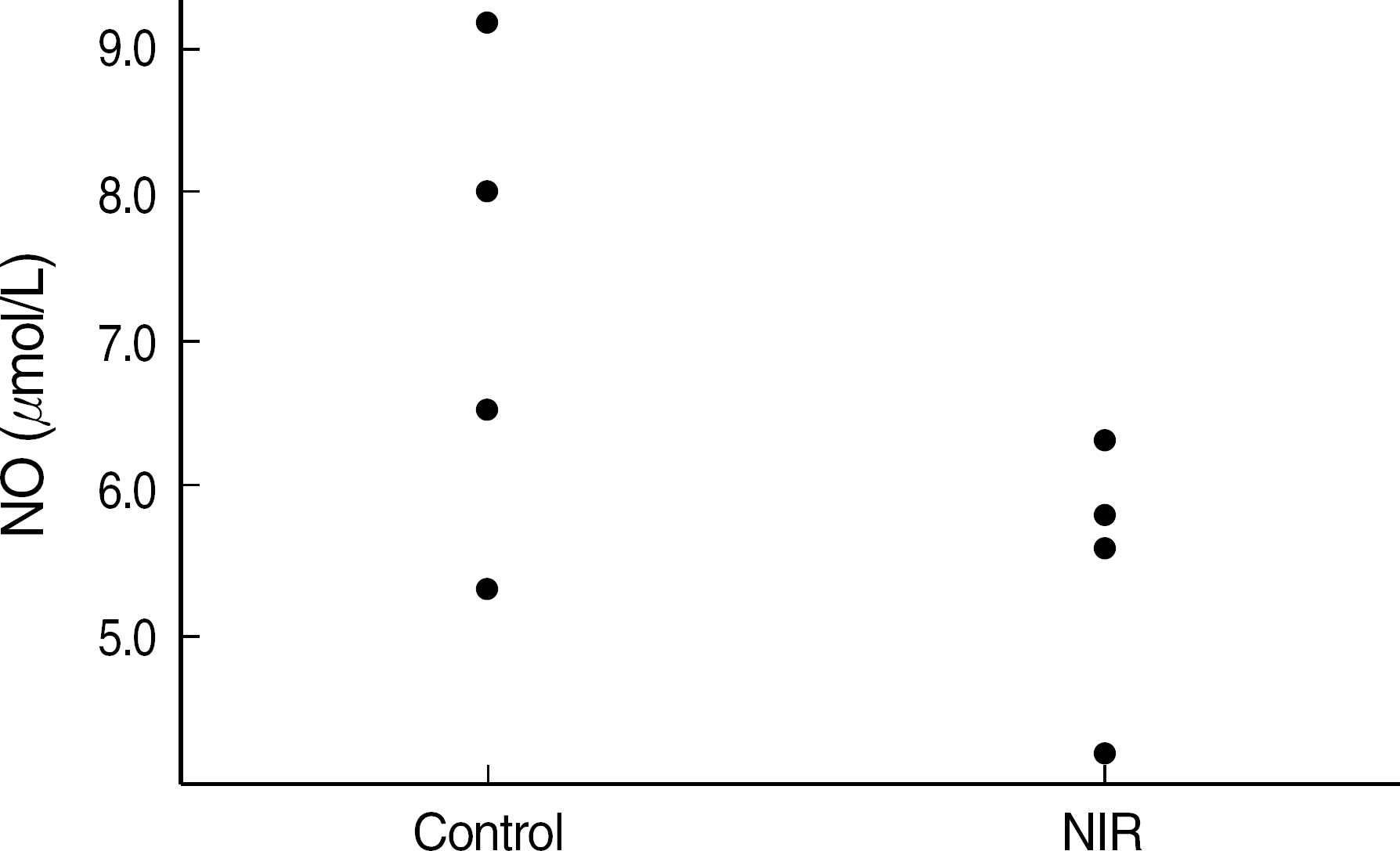
Fig. 5.
LPS (10 mcg/mL) stimulated NIH3T3 cells show green fluorescence after Immunofluorescence staining of iNOS-FITC antibody (epi-fluoresence microscopy, NiKon, micro-FXA ×400). Abbreviations: See Table 1.
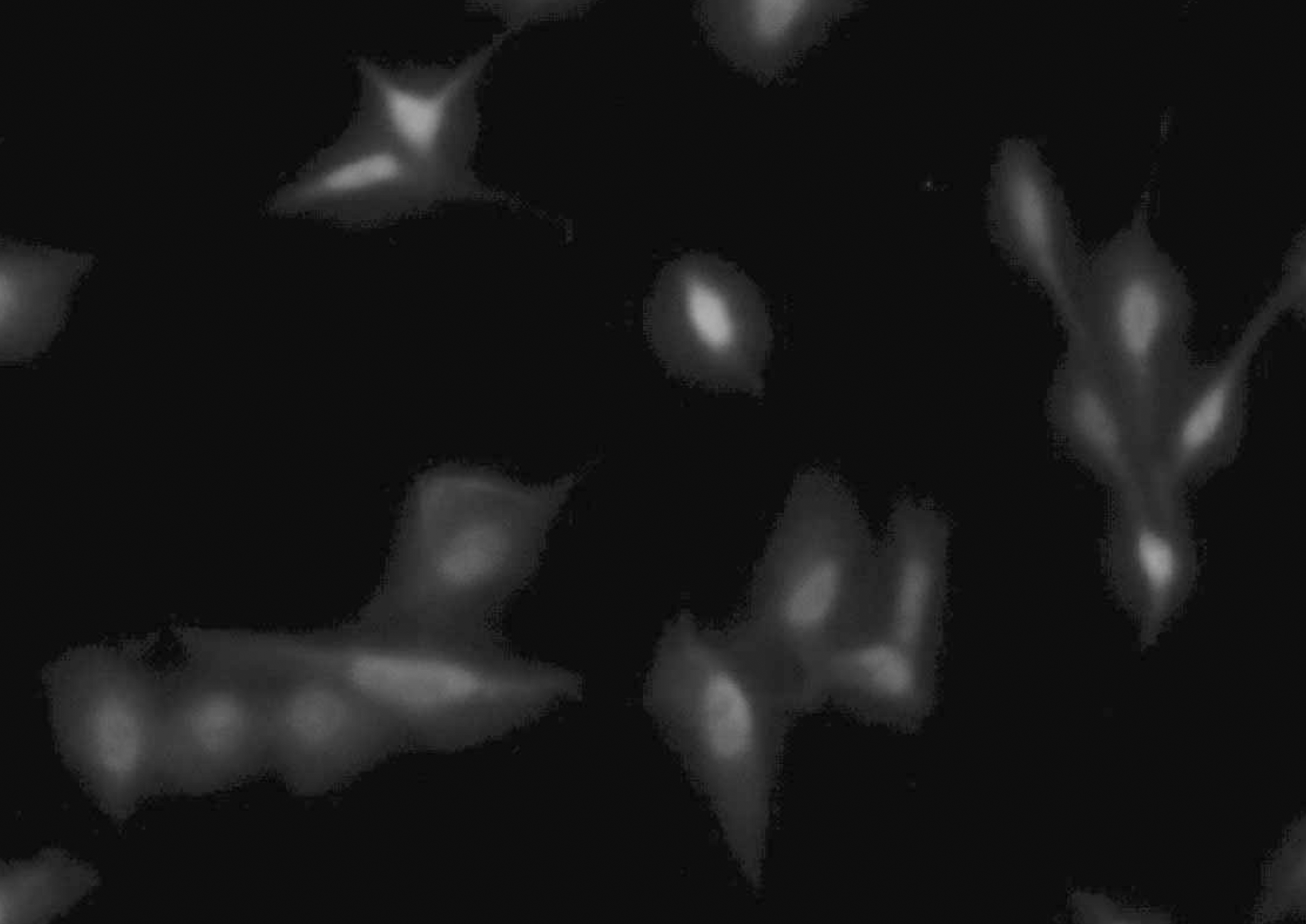
Table 1.
Result of immunofluorescence staining of inducible nitric oxide synthase expression of the NIH3T3 cells




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download