Abstract
Background:
There is no guideline for the appropriate wavelength at which to measure the optical density (OD) value in broth microdilution antifungal susceptibility testing, although a spectrophotometric reading method is commonly used. The present study aimed to analyze the difference in the OD values over the range of visible light and to ascertain the optimal wavelength for the spectrophotometric method of microdilution testing.
Methods:
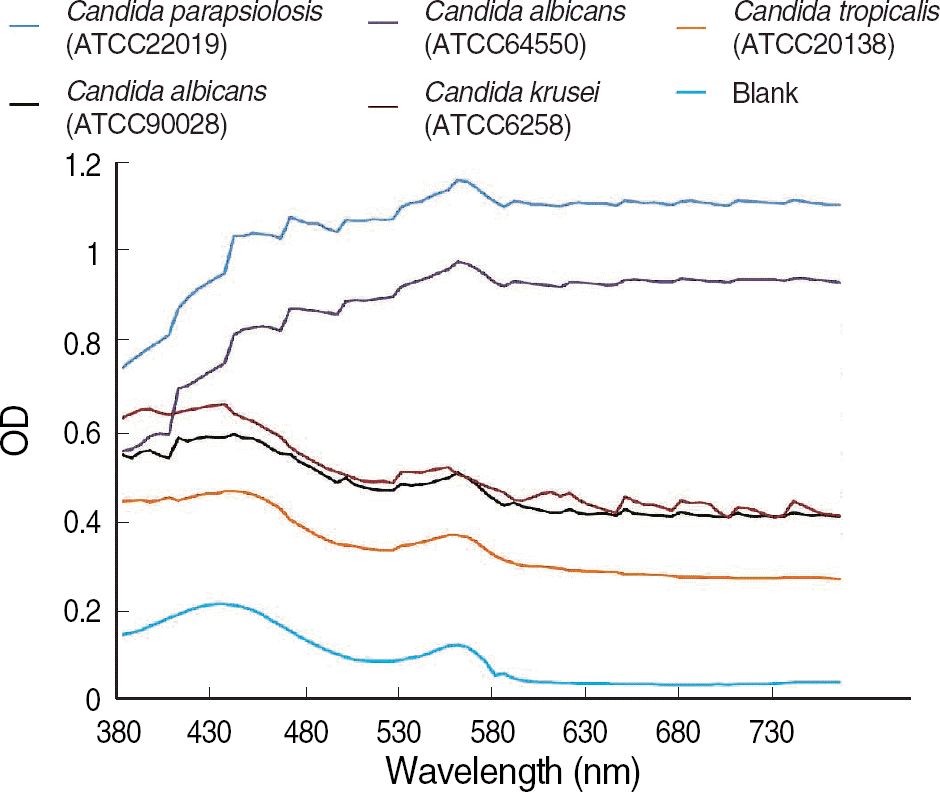
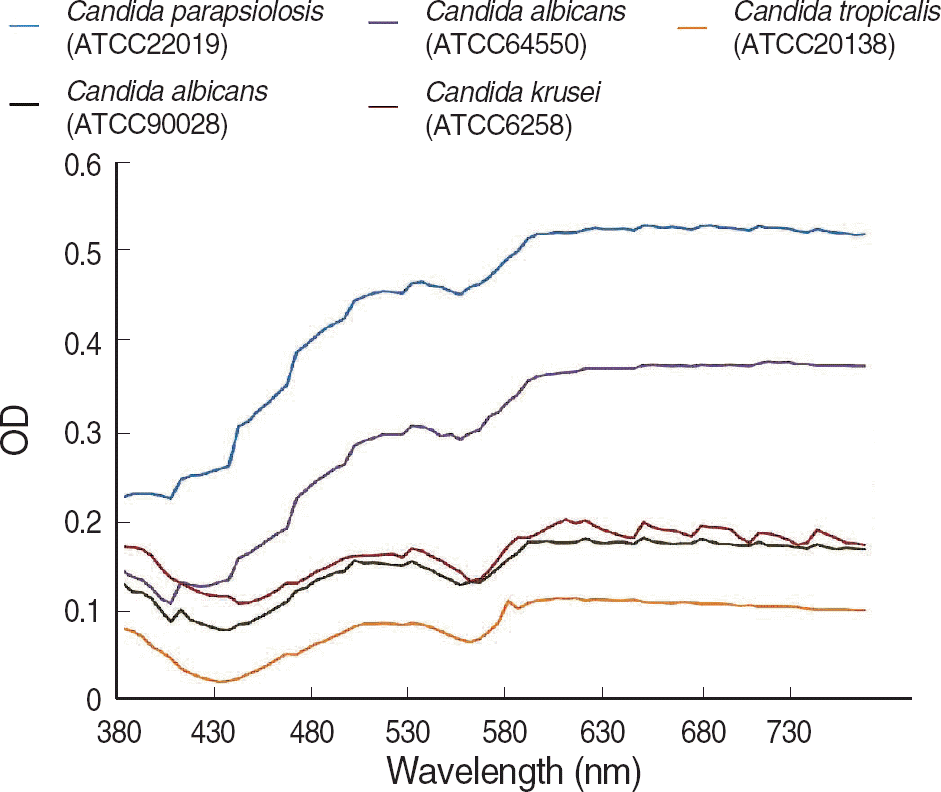
We measured the OD of background blank controls of broth medium, antifungal agents, and inocula of five type strains using a Synergy HT multi-detection microplate reader at 5-nm intervals from 380 nm to 760 nm. We also estimated the OD differences between the 50% of growth control and blank control.
Results:
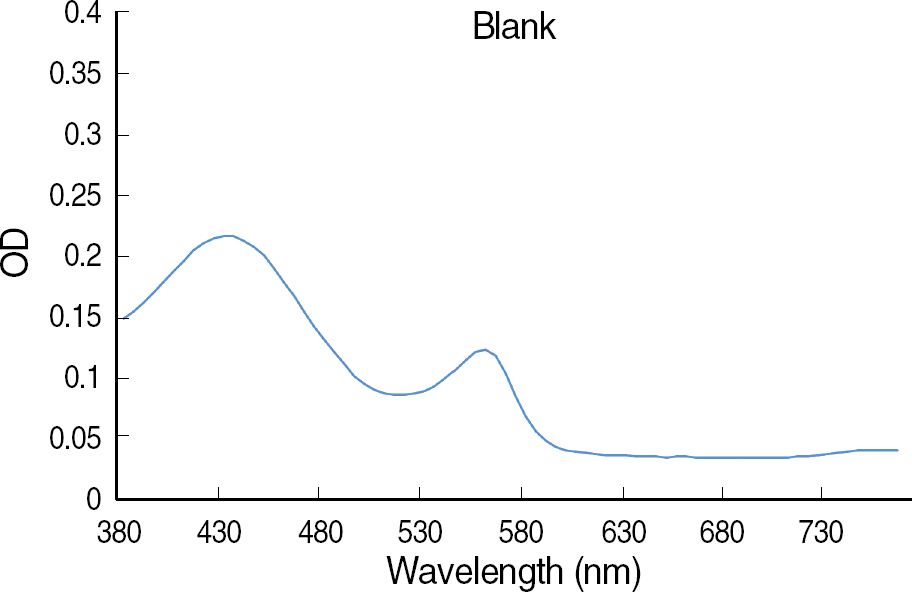
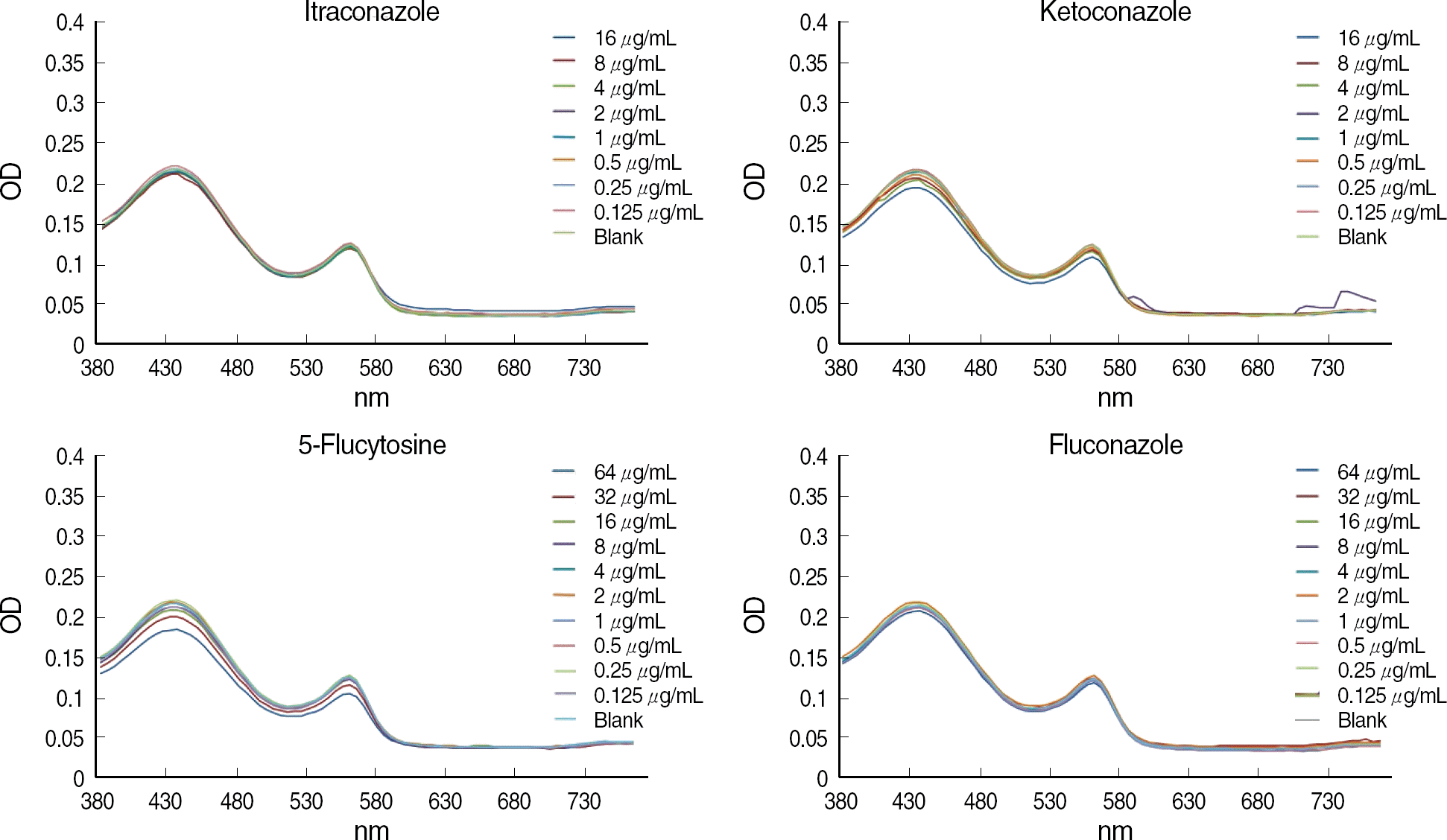
The OD of the blank control showed a parabola shape with two peaks and steadily decreased at longer wavelengths. The curves of the antifungal agent were similar to those of blank controls, and the influence of each antifungal agent on the OD was minimal. For the difference in OD between 50% of growth control and the blank control, the curve was the opposite of the blank control, and the OD increased steadily at the wavelengths above 600 nm.
Go to : 
REFERENCES
1.Neely MN., Ghannoum MA. The exciting future of antifungal therapy. Eur J Clin Microbiol Infect Dis. 2000. 19:897–914.

2.Pfaller MA., Jones RN., Doern GV., Sader HS., Messer SA., Houston A, et al. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob Agents Chemother. 2000. 44:747–51.
3.Rex JH., Pfaller MA., Walsh TJ., Chaturvedi V., Espinel-Ingroff A., Ghannoum MA, et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev. 2001. 14:643–58.

4.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-second edition. CLSI document M27-A2. 2nd ed.Wayne, PA: Clinical and Laboratory Standards Institute;2002.
5.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition. CLSI document M27-A3. 3rd ed.Wayne, PA: Clinical and Laboratory Standards Institute;2008.
6.Pina-Vaz C., Costa-de-Oliveira S., Rodrigues AG., Espinel-Ingroff A. Comparison of two probes for testing susceptibilities of pathogenic yeasts to voriconazole, itraconazole, and caspofungin by flow cytometry. J Clin Microbiol. 2005. 43:4674–9.

7.Chaturvedi V., Ramani R., Pfaller MA. Collaborative study of the NCCLS and flow cytometry methods for antifungal susceptibility testing of Candida albicans. J Clin Microbiol. 2004. 42:2249–51.
8.Pfaller MA., Boyken L., Messer SA., Tendolkar S., Hollis RJ., Diekema DJ. Evaluation of the etest method using Mueller-Hinton agar with glucose and methylene blue for determining amphotericin B MICs for 4,936 clinical isolates of Candida species. J Clin Microbiol. 2004. 42:4977–9.
9.Park JY., Shin JH., Uh Y., Kim EC., Kee SJ., Kim SH, et al. In vitro amphotericin B susceptibility of Korean bloodstream yeast isolates assessed by the CLSI broth microdilution method, Etest, and Minimum fungicidal concentration test. Korean J Lab Med. 2008. 28:346–52. (박지영, 신종희, 어영, 김의종, 기승정, 김수현등. CLSI 액체배지미량희석 법, Etest 및검사에의한국내혈 액에서 분리된 효모균의 시험관 내 Minimum Fungicidal Concentration 검사에의한국내혈 액에서 분리된 효모균의 시험관 내 Amphotericin B 내성 성적. 대한진 단검사의학회지 2008;28:346-52.).

10.Wanger A., Mills K., Nelson PW., Rex JH. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995. 39:2520–2.
11.Lopez-Jodra O., Torres-Rodriguez JM., Mendez-Vasquez R., Ribas-Forcadell E., Morera-Lopez Y., Baro-Tomas T, et al. In vitro susceptibility of Cryptococcus neoformans isolates to five antifungal drugs using a colorimetric system and the reference microbroth method. J Antimicrob Chemother. 2000. 45:645–9.
12.Shin JH., Choi JC., Lee JN., Kim HH., Lee EY., Chang CL. Evaluation of a colorimetric antifungal susceptibility test by using 2,3-diphenyl-5-thienyl-(2)-tetrazolium chloride. Antimicrob Agents Chemother. 2004. 48:4457–9.

13.To WK., Fothergill AW., Rinaldi MG. Comparative evaluation of macrodilution and alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1995. 33:2660–4.

14.Pfaller MA., Messer SA., Coffmann S. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J Clin Microbiol. 1995. 33:1094–7.

15.Arthington-Skaggs BA., Lee-Yang W., Ciblak MA., Frade JP., Brandt ME., Hajjeh RA, et al. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother. 2002. 46:2477–81.
16.Pfaller MA., Rex JH., Rinaldi MG. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997. 24:776–84.

17.Rex JH., Pfaller MA., Galgiani JN., Bartlett MS., Espinel-Ingroff A., Ghannoum MA, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997. 24:235–47.
18.Hawser SP., Norris H., Jessup CJ., Ghannoum MA. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998. 36:1450–2.
19.Yi JY., Shin JH., Lee K., Yong D., Chae MJ., Suh SP, et al. Evaluation of spectrophotometric broth microdilution method to determine the fluconazole MIC of the Candida species. Korean J Lab Med. 2002. 22:253–9. (이지연, 신종희, 이경원, 용동은, 채명종, 서순팔 등. Candida 균 종의 Fluconazole 감수성검사를위한 Spectrophotometric Broth Microdilution 법의평가.대한진단검사의학회지 2002; 22:253–9.).
20.Chryssanthou E., Cuenca-Estrella M. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J Clin Microbiol. 2002. 40:3841–4.

21.Moore CB., Walls CM., Denning DW. Comparison of three methods for in vitro susceptibility testing of Candida species with flucytosine. J Antimicrob Chemother. 2003. 51:297–304.
22.Cuenca-Estrella M., Lee-Yang W., Ciblak MA., Arthington-Skaggs BA., Mellado E., Warnock DW, et al. Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of Candida species. Antimicrob Agents Chemother. 2002. 46:3644–7.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download