Abstract
Background:
Bacteroides fragilis group organisms are the most frequently isolated anaerobes in human infections. Increasing resistance to various antimicrobial agents is a significant problem in choosing appropriate antimicrobial agents to treat anaerobic infections. Periodic monitoring of the regional resistance trends of B. fragilis group isolates is needed.
Methods:
A total of 466 nonduplicate clinical isolates of B. fragilis group organisms (276 B. fragilis, 106 Bacteroides thetaiotaomicron, and 84 other B. fragilis group organisms) were collected during the 8-yr period from 1997 to 2004 in a Korean university hospital. Minimum inhibitory concentrations to various antimicrobial agents were determined by the CLSI agar dilution method.
Results:
Eight isolates were resistant to imipenem. Additionally, the resistance rates to cefotetan were decreased in B. thetaiotaomicron, while those for clindamycin were significantly increased compared to the rates found in previous studies. Depending on species, resistance rates were 1-4% for imipenem, 1-6% for piperacillin-tazobactam, 4-11% for cefoxitin, 33-49% for piperacillin, 14-60% for cefotetan, and 51-76% for clindamycin. No isolates were resistant to chloramphenicol or metronidazole.
Conclusions:
Piperacillin-tazobactam, cefoxitin, imipenem, chloramphenicol, and metronidazole are still active against B. fragilis group isolates, while clindamycin no longer has a value as an empirical therapeutic agent in Korea. Furthermore, this study identified the first imipenem-resistant B. fragilis group isolates in Korea.
Go to : 
REFERENCES
1.Citron DM., Poxton IR., Baron EJ. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and other anaerobic gram-negative rods. Murray PR, Baron EJ, editors. Manual of Clinical Microbiology. 9th ed.Washington D.C.: ASM Press;2007. p. 911–32.
3.Lassmann B., Gustafson DR., Wood CM., Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007. 44:895–900.

4.Redondo MC., Arbo MD., Grindlinger J., Snydman DR. Attributable mortality of bacteremia associated with the Bacteroides fragilis group. Clin Infect Dis. 1995. 20:1492–6.
5.Behra-Miellet J., Calvet L., Mory F., Muller C., Chomarat M., Bezian MC, et al. Antibiotic resistance among anaerobic Gram-negative bacilli: lessons from a French multicentric survey. Anaerobe. 2003. 9:105–11.

6.Snydman DR., Jacobus NV., McDermott LA., Ruthazer R., Golan Y., Goldstein EJ, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007. 51:1649–55.
7.Nguyen MH., Yu VL., Morris AJ., McDermott L., Wagener MW., Harrell L, et al. Antimicrobial resistance and clinical outcome of Bacteroides bacteremia: findings of a multicenter prospective observational trial. Clin Infect Dis. 2000. 30:870–6.
8.Lee K., Chong Y., Jeong SH., Xu XS., Kwon OH. Emerging resistance of anaerobic bacteria to antimicrobial agents in South Korea. Clin Infect Dis. 1996. 23(S1):73–7.

9.Lee K., Shin HB., Chong Y. Antimicrobial resistance patterns of Bacteroides fragilis group organisms in Korea. Yonsei Med J. 1998. 39:578–86.
10.Clinical Laboratory Standards Institute. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standards. M11-A6. 6th ed.Wayne, PA: Clinical Laboratory Standards Institute;2004.
11.Snydman DR., Jacobus NV., McDermott LA., Ruthazer R., Goldstein EJ., Finegold SM, et al. National survey on the susceptibility of Bacteroides Fragilis Group: report and analysis of trends for 1997-2000. Clin Infect Dis. 2002. 35(S1):126–34.
12.Holdeman LV, Cato EP, editors. Anaerobic laboratory manual. 4th ed.Blacksburg, VA: Virginia Polytechnic Institute and State University;1977.
13.Summanen P, Baron EJ, editors. Wadsworth anaerobic bacteriology manual. 5th ed.Belmont, CA: Star Publishing;1993.
14.Goldstein EJ., Snydman DR. Intra-abdominal infections: review of the bacteriology, antimicrobial susceptibility and the role of ertapenem in their therapy. J Antimicrob Chemother. 2004. 53(S2):ii. 29–36.

15.Soki J., Edwards R., Hedberg M., Fang H., Nagy E., Nord CE. Examination of cfiA-mediated carbapenem resistance in Bacteroides fragilis strains from a European antibiotic susceptibility survey. Int J Antimicrob Agents. 2006. 28:497–502.
16.Betriu C., Culebras E., Gomez M., Lopez F., Rodriguez-Avial I., Picazo JJ. Resistance trends of the Bacteroides fragilis group over a 10-year period, 1997 to 2006, in Madrid, Spain. Antimicrob Agents Chemother. 2008. 52:2686–90.
17.Snydman DR., Jacobus NV., McDermott LA. In vitro activities of doripenem, a new broad-spectrum carbapenem, against recently collected clinical anaerobic isolates, with emphasis on the Bacteroides fragilis group. Antimicrob Agents Chemother. 2008. 52:4492–6.
18.Podglajen I., Breuil J., Casin I., Collatz E. Genotypic identification of two groups within the species Bacteroides fragilis by ribotyping and by analysis of PCR-generated fragment patterns and insertion sequence content. J Bacteriol. 1995. 177:5270–5.
19.Khushi T., Payne DJ., Fosberry A., Reading C. Production of metal dependent beta-lactamases by clinical strains of Bacteroides fragilis isolated before 1987. J Antimicrob Chemother. 1996. 37:345–50.
20.Walsh TR., Onken A., Haldorsen B., Toleman MA., Sundsfjord A. Characterization of a carbapenemase-producing clinical isolate of Bacteroides fragilis in Scandinavia: genetic analysis of a unique insertion sequence. Scand J Infect Dis. 2005. 37:676–9.
21.Rasmussen BA., Bush K., Tally FP. Antimicrobial resistance in Bacteroides. Clin Infect Dis. 1993. 16(S4):390–400.
22.Stearne LE., van Boxtel D., Lemmens N., Goessens WH., Mouton JW., Gyssens IC. Comparative study of the effects of ceftizoxime, piperacillin, and piperacillin-tazobactam concentrations on antibacterial activity and selection of antibiotic-resistant mutants of Enterobacter cloacae and Bacteroides fragilis in vitro and in vivo in mixed-infection abscesses. Antimicrob Agents Chemother. 2004. 48:1688–98.
23.Lee K., Jang IH., Kim YJ., Chong Y. In vitro susceptibilities of the Bacteroides fragilis group to 14 antimicrobial agents in Korea. Antimicrob Agents Chemother. 1992. 36:195–7.
24.Liu CY., Huang YT., Liao CH., Yen LC., Lin HY., Hsueh PR. Increasing trends in antimicrobial resistance among clinically important anaerobes and Bacteroides fragilis isolates causing nosocomial infections: emerging resistance to carbapenems. Antimicrob Agents Chemother. 2008. 52:3161–8.
25.Rasmussen BA., Bush K., Tally FP. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997. 24(S1):110–20.

26.Brazier JS., Stubbs SL., Duerden BI. Metronidazole resistance among clinical isolates belonging to the Bacteroides fragilis group: time to be concerned? J Antimicrob Chemother. 1999. 44:580–1.
27.Dubreuil L., Breuil J., Dublanchet A., Sedallian A. Survey of the susceptibility patterns of Bacteroides fragilis group strains in France from 1977 to 1992. Eur J Clin Microbiol Infect Dis. 1992. 11:1094–9.
28.Suata K., Watanabe K., Ueno K., Homma M. Antimicrobial susceptibility patterns and resistance transferability among Bacteroides fragilis group isolates from patients with appendicitis in Bali, Indonesia. Clin Infect Dis. 1993. 16:561–6.
Go to : 
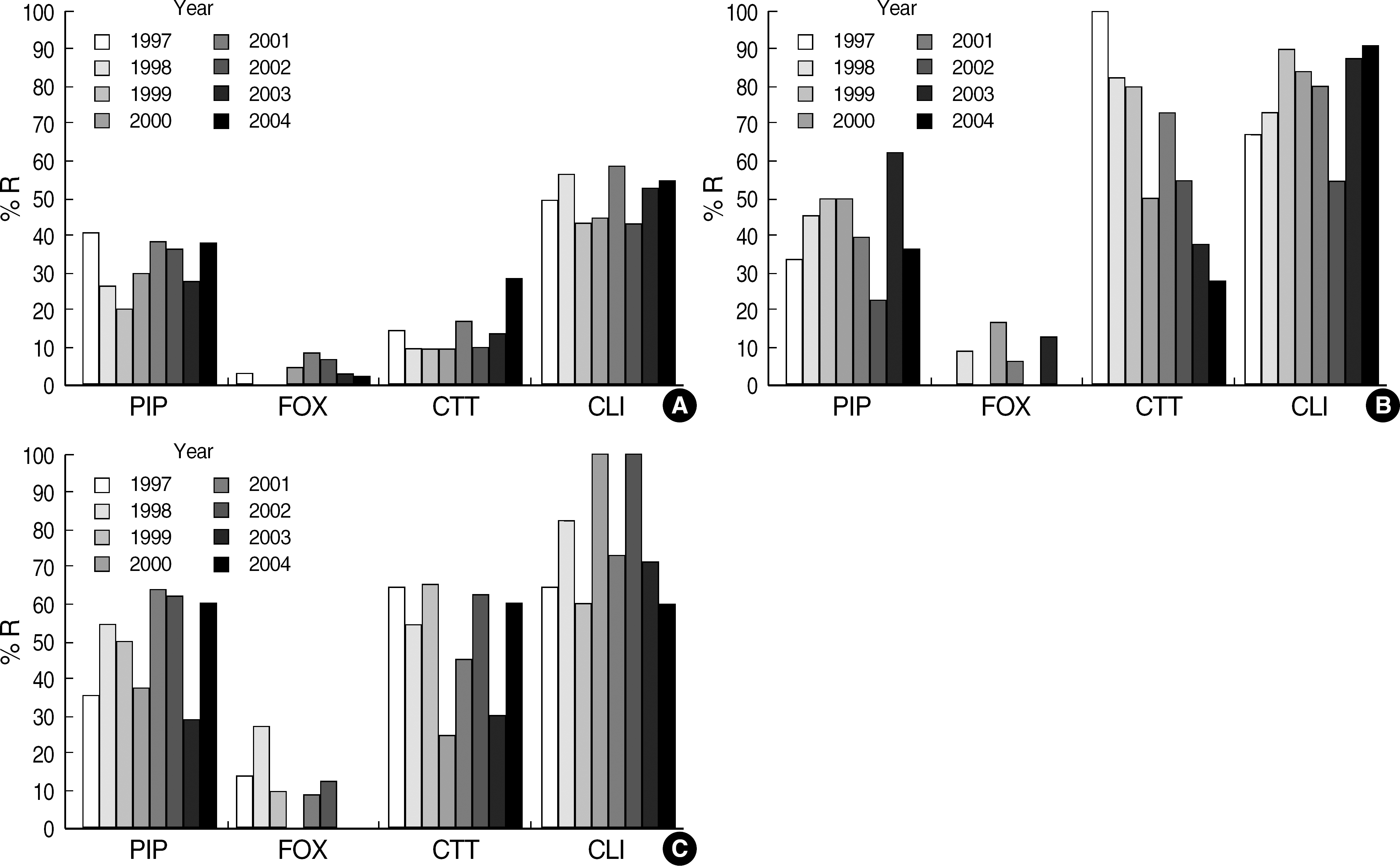 | Fig. 1.Change in percent resistance of Bacteroides fragilis group organisms isolated from 1997 to 2004. (A) B. fragilis, (B) B. thetaiotaomicron, (C) Other Bacteroides spp.
Abbreviations: PIP, piperacillin; FOX, cefoxitin; CTT, cefotetan; CLI, clindamycin.
|
Table 1.
Distribution of the species within the Bacteroides fragilis group organisms isolated from 1997 to 2004
Table 2.
Antimicrobial susceptibility of Bacteroides fragilis group organisms isolated from 1997 to 2004




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download