Abstract
Chimerism testing permits early prediction and documentation of successful engraftment, and also facilitates detection of impending graft rejection. In this study, we serially monitored chimerism status by short tandem repeat-based PCR in nucleated cells (NC), T cells and natural killer (NK) cells after myeloablative allogeneic stem cell transplantation (SCT). Four patients with myeloid malignancies showed discrepant chimerism results among those three fractions. Three patients had mixed chimerism (MC) of donor/host T cells at a time point around the onset of chronic graft-versus-host disease (GVHD). In two patients with disease relapse, MC of NK cells preceded a morphological relapse or NK cells showed a higher percentage of patient cells compared to NC. Therefore, our study shows that chimerism analysis in lineage-specific cells might be useful in predicting clinical outcome after allogeneic SCT in certain patients.
REFERENCES
1.Weng L., Dyson J., Dazzi F. Low-intensity transplant regimens facilitate recruitment of donor-specific regulatory T cells that promote hematopoietic engraftment. Proc Natl Acad Sci USA. 2007. 104:8415–20.

2.Lobashevsky AL., Senkbeil RW., Townsend JE., Mink CA., Thomas JM. Quantitative analysis of chimerism using a short tandem repeat method on a fluorescent automated DNA sequencer. Clin Lab Haematol. 2006. 28:40–9.

3.Lassaletta A., Ramírez M., Montero JM., González-Vicent M., Balas A., Madero L, et al. Full donor chimerism by day 30 after allogeneic peripheral blood progenitor cell transplantation is associated with a low risk of relapse in pediatric patients with hematological malignancies. Leukemia. 2005. 19:504–6.

4.Bader P., Niethammer D., Willasch A., Kreyenberg H., Klingebiel T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant. 2005. 35:107–19.

5.Baron F., Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006. 20:1690–700.

6.Fernández-Avilés F., Urbano-Ispizua A., Aymerich M., Colomer D., Rovira M., Martínez C, et al. Serial quantification of lymphoid and myeloid mixed chimerism using multiplex PCR amplification of short tandem repeat-markers predicts graft rejection and relapse, respectively, after allogeneic transplantation of CD34+ selected cells from peripheral blood. Leukemia. 2003. 17:613–20.

7.Bader P., Stoll K., Huber S., Geiselhart A., Handgretinger R., Niemeyer C, et al. Characterization of lineage-specific chimaerism in patients with acute leukaemia and myelodysplastic syndrome after allogeneic stem cell transplantation before and after relapse. Br J Haematol. 2000. 108:761–78.

8.Huisman C., de Weger RA., de Vries L., Tilanus MG., Verdonck LF. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant. 2007. 39:285–91.

9.Zeiser R., Spyridonidis A., Wäsch R., Ihorst G., Grüllich C., Bertz H, et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2005. 19:814–21.

10.Miura Y., Tanaka J., Toubai T., Tsutsumi Y., Kato N., Hirate D, et al. Analysis of donor-type chimerism in lineage-specific cell populations after allogeneic myeloablative and non-myeloablative stem cell transplantation. Bone Marrow Transplant. 2006. 37:837–43.

11.Matthes-Martin S., Lion T., Haas OA., Frommlet F., Daxberger H., König M, et al. Lineage-specific chimaerism after stem cell transplantation in children following reduced intensity conditioning: potential predictive value of NK cell chimaerism for late graft rejection. Leukemia. 2003. 17:1934–42.

12.Michallet AS., Fürst S., Le QH., Dubois V., Praire A., Nicolini F, et al. Impact of chimaerism analysis and kinetics on allogeneic haematopoietic stem cell transplantation outcome after conventional and reduced-intensity conditioning regimens. Br J Haematol. 2005. 128:676–89.

13.Mattsson J., Uzunel M., Brune M., Hentschke P., Barkholt L., Stierner U, et al. Mixed chimaerism is common at the time of acute graft-versus-host disease and disease response in patients receiving non-myeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol. 2001. 115:935–44.

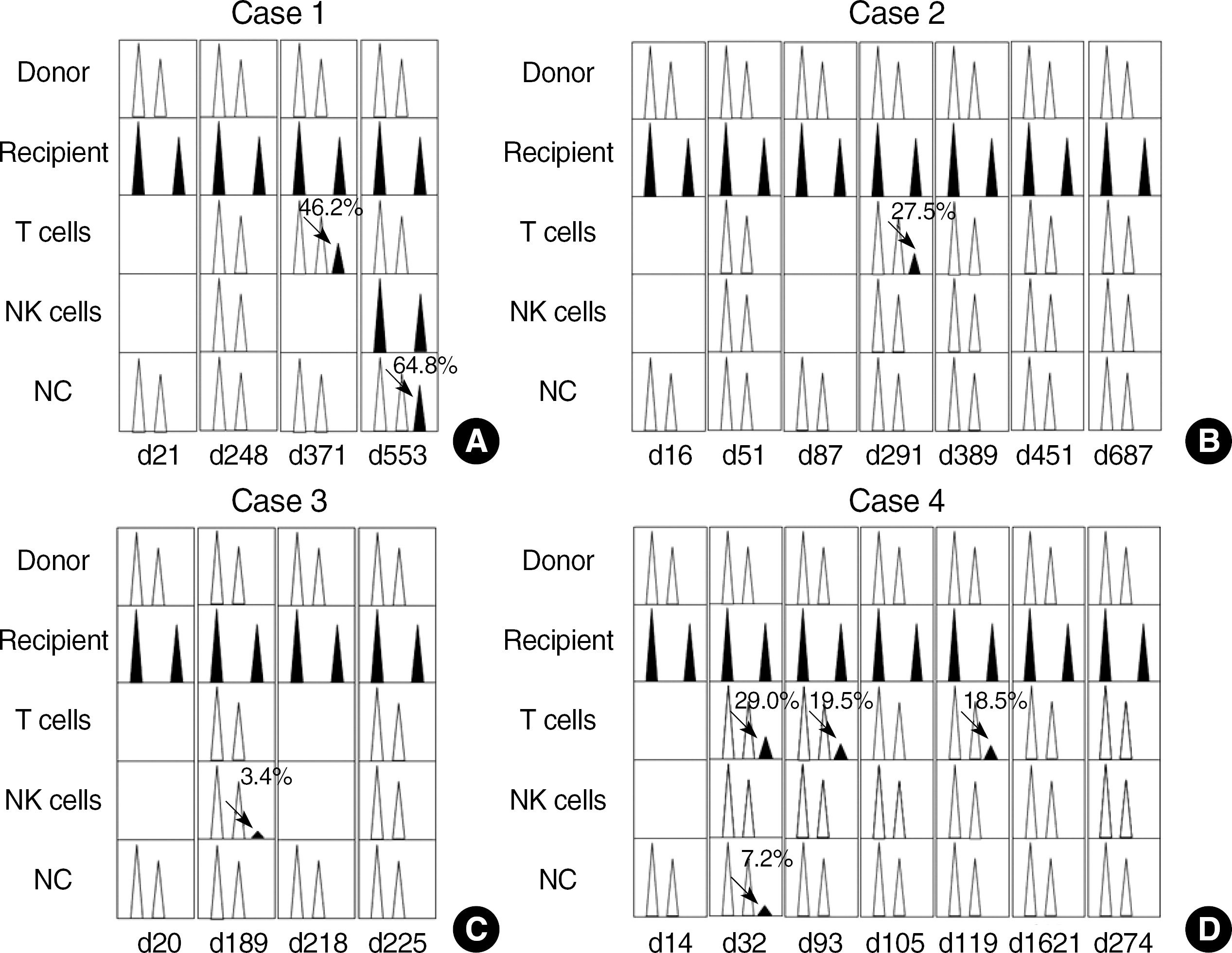
Fig. 1.
Schematic presentation of serial lineage-specific chimerism data in four patients. (A) 46.2% MC of T cells on day 371 and 100% patient cells of NK cells and 64.8% MC of NC on day 553, respectively (arrows). (B) MC of T cells alone on day 291 (arrow). (C) On day 189, only NK cells showed 3.4% MC (arrow). (D) MC of T cells showing 29.0%, 19.5%, and 18.5% of patient cells on day 32, 93, and 119, respectively. NC showed 7.2% of patient cells briefly at day 32 (arrows).
Abbreviations: MC, mixed chimerism; NC, nucleated cells; NK, natural killer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download