Abstract
Background:
We analyzed T cell receptor β chain (TCRB) gene to investigate the presence of putative T cell clones and its clinicopathologic implications in Korean patients with aplastic anemia (AA).
Methods:
Twenty-nine bone marrow specimens were collected from 20 AA patients, 19 specimens from initial diagnosis and 10 from follow-up. T cell clonality assay was performed using IdentiClone™ TCRB Gene Clonality Assay kit (InVivoScirbe Technology, USA) and automatic genetic analyzer. Patients' clinical information and laboratory parameters were also analyzed.
Results:
Five patients had definitive underlying factors related with aplastic anemia, such as hepatitis B virus (4 cases) and benzene exposure (1 case). Putative T cell clones were detected in bone marrow specimens of 11 (58%) out of 19 patients at diagnosis. The location of putative T cell clones of TCRB gene (diversity region, Dβ; joining region, Jβ; variable region, Vβ) was distributed in Dβ2+ Jβ2 (6 cases), Dβ1+Jβ1 (3 cases), Vβ+Jβ1 (2 cases), and Dβ1+Jβ2 (2 cases). Interestingly, among seven patients who underwent stem cell transplantation, five patients with no T cell clones detected at diagnosis developed new T cell clones during the follow-up.
REFERENCES
2.Young NS., Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008. 93:489–92.

4.Walne AJ., Dokal I. Telomerase dysfunction and dyskeratosis congenita. Cytotechnology. 2004. 45:13–22.

5.Rosenfeld SJ., Kimball J., Vining D., Young NS. Intensive immuno-suppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995. 85:3058–65.

6.Zeng W., Nakao S., Takamatsu H., Yachie A., Takami ., Kondo Y, et al. Characterization of T-cell repertoire of the bone marrow in immune-mediated aplastic anemia: evidence for the involvement of antigen-driven T-cell response in cyclosporine-dependent aplastic anemia. Blood. 1999. 93:3008–16.

7.Kook H., Risitano AM., Zeng W., Wlodarski M., Lottemann C., Nakamura R, et al. Changes in T-cell receptor VB repertoire in aplastic anemia: effects of different immunosuppressive regimens. Blood. 2002. 99:3668–75.

8.Young NS. Hematopoietic cell destruction by immune mechanisms in acquired aplastic anemia. Semin Hematol. 2000. 37:3–14.

9.Wlodarski MW., Gondek LP., Nearman ZP., Plasilova M., Kalaycio M., Hsi ED, et al. Molecular strategies for detection and quantitation of clonal cytotoxic T-cell responses in aplastic anemia and myelodysplastic syndrome. Blood. 2006. 108:2632–41.

10.Risitano AM., Maciejewski JP., Green S., Plasilova M., Zeng W., Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004. 364:355–64.
11.Plasilova M., Risitano A., Maciejewski JP. Application of the molecular analysis of the T-Cell receptor repertoire in the study of immune-mediated hematologic diseases. Hematology. 2003. 8:173–81.

12.Maciejewski JP., O'Keefe C., Gondek L., Tiu R. Immune-mediated bone marrow failure syndromes of progenitor and stem cells: molecular analysis of cytotoxic T cell clones. Folia Histochem Cytobiol. 2007. 45:5–14.
13.Risitano AM., Kook H., Zeng W., Chen G., Young NS., Maciejewski JP. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002. 100:178–83.
14.Abbaas AK, Lichtman AH, editors. Cellular and moleclular immunology. 6th ed.Philadelphia: Saunders;2007. p. 160–9.
15.van Dongen JJ., Langerak AW., Bruggemann M., Evans PA., Hummel M., Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003. 17:2257–317.

16.Sandberg Y., van Gastel-Mol EJ., Verhaaf B., Lam KH., van Dongen JJ., Langerak AW. BIOMED-2 multiplex immunoglobulin/T-cell receptor polymerase chain reaction protocols can reliably replace Southern blot analysis in routine clonality diagnostics. J Mol Diagn. 2005. 7:495–503.

17.Camitta BM., Thomas ED., Nathan DG., Santos G., Gordon-Smith EC., Gale RP, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976. 48:63–70.

18.Nakao S., Takamatsu H., Chuhjo T., Ueda M., Shiobara S., Matsuda T, et al. Identification of a specific HLA class II haplotype strongly associated with susceptibility to cyclosporine-dependent aplastic anemia. Blood. 1994. 84:4257–61.

19.Maciejewski JP., Follmann D., Nakamura R., Saunthararajah Y., Rivera CE., Simonis T, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001. 98:3513–9.

20.Demeter J., Messer G., Schrezenmeier H. Clinical relevance of the TNF-alpha promoter/enhancer polymorphism in patients with aplastic anemia. Ann Hematol. 2002. 81:566–9.

21.Dufour C., Capasso M., Svahn J., Marrone A., Haupt R., Bacigalupo A, et al. Homozygosis for (12) CA repeats in the first intron of the human IFN-gamma gene is significantly associated with the risk of aplastic anaemia in Caucasian population. Br J Haematol. 2004. 126:682–5.
22.Gidvani V., Ramkissoon S., Sloand EM., Young NS. Cytokine gene polymorphisms in acquired bone marrow failure. Am J Hematol. 2007. 82:721–4.

23.Calado RT., Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008. 111:4446–55.

24.Solomou EE., Keyvanfar K., Young NS. T-bet, a Th1 transcription factor, is up-regulated in T cells from patients with aplastic anemia. Blood. 2006. 107:3983–91.

25.Solomou EE., Rezvani K., Mielke S., Malide D., Keyvanfar K., Visconte V, et al. Deficient CD4+CD25+FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007. 110:1603–6.
Fig. 1.
Schematic diagram of strategy for T cell clonality assay. A representative rearranged T cell receptor beta gene on chromosome 7 (7q35) shows the approximate placement of the upstream and downstream DNA primers. The numbers of primers and their specificity are listed for master mix tubes A, B and C.
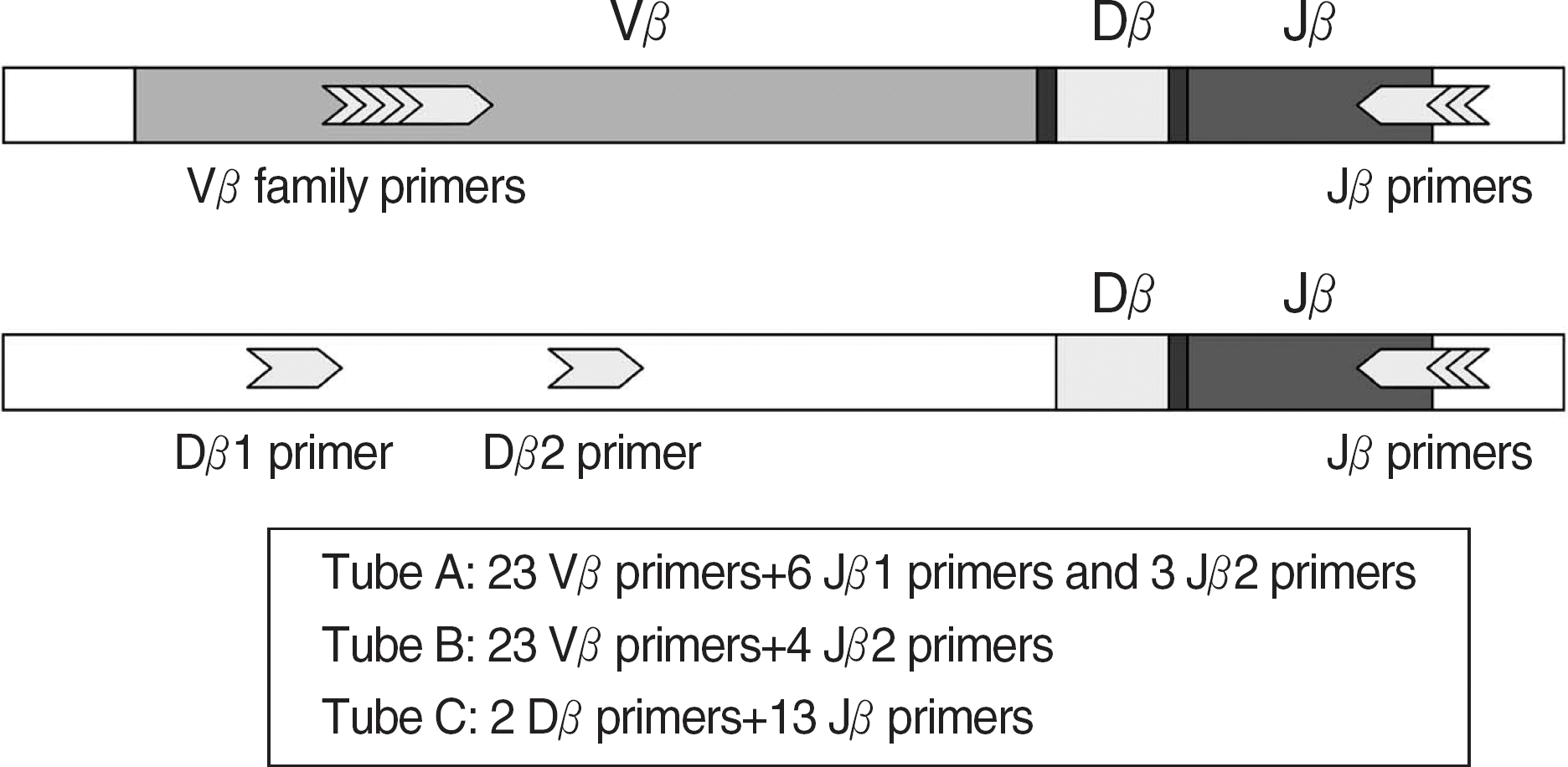
Fig. 2.
Capillary electrophoretogram of amplified T-cell receptor beta chain (TCRB) gene. Positive peaks (presence of clonal cell population) are observed in IVS-0004 positive control DNA at 295 base pairs (bp) (A), and patient No. 13 at 21 days after bone marrow transplantation in TCRB variable and joining region (B). Typical polyclonal peaks are observed in Patient No. 20 at 240-285 bp (C). The definition of positive peaks was at least three times the amplitude of the third largest peak in the polyclonal background within the valid size range.
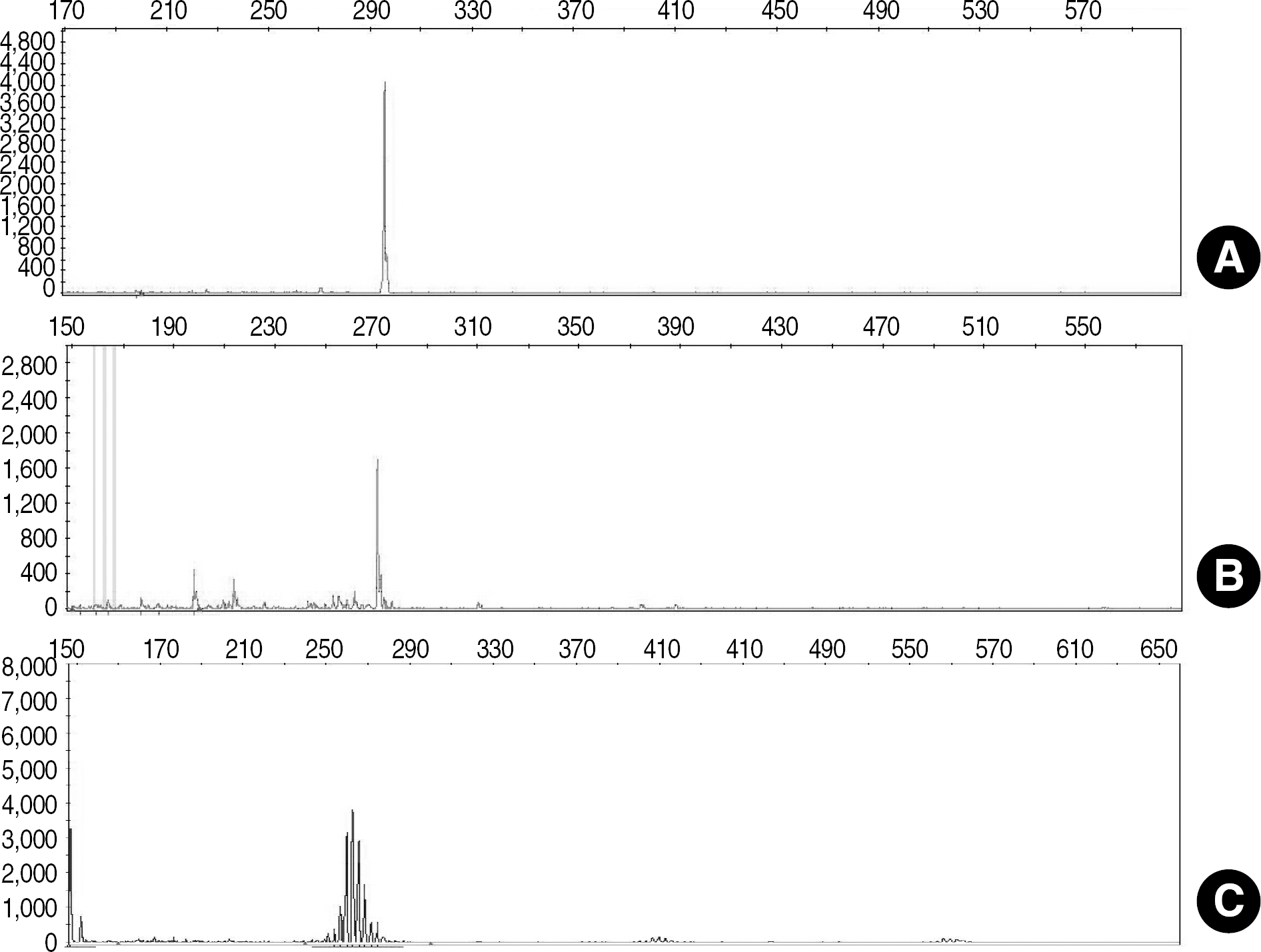
Table 1.
Patient characteristics and laboratory data
| Patient No. | Age | Sex | Underlying factor | BM cellularity (%) | WBC (ANC) (×103/μL) | Hemoglobin (g/dL) | Platelet (×103/μL) | Corrected reticulocyte count (%) | Severity | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1∗ | 35 | M | HBV, benzene | 5 | 1,900 (600) | 7.4 | 10 | 1.5 | IST | |
| 2 | 38 | M | - | 10 | 940 (400) | 4.4 | 28 | 0.1 | Severe | IST |
| 3 | 36 | M | - | 10 | 2,440 (100) | 10.0 | 2 | 0.4 | Very severe | BMT |
| 4 | 21 | M | HBV | 10 | 400 (70) | 7.8 | 36 | 0.1 | Very severe | PBSCT |
| 5 | 25 | F | - | 10 | 600 (80) | 7.0 | 52 | 0.1 | Very severe | IST |
| 6 | 33 | M | HBV | 10 | 1,700 (360) | 7.3 | 24 | 0.4 | Severe | |
| 7 | 50 | F | - | 10 | 2,000 (70) | 8.7 | 7 | 0.4 | Very severe | BMT |
| 8 | 69 | M | - | 10 | 1,700 (160) | 6.0 | 33 | 0.1 | Very severe | |
| 9 | 28 | F | - | 10 | 2,400 (620) | 6.3 | 10 | 0.4 | Severe | BMT |
| 10 | 27 | F | - | 10 | 2,000 (390) | 5.7 | 9 | 0.1 | Severe | BMT |
| 11 | 73 | F | - | 10 | 3,100 (500) | 5.5 | 5 | 0.2 | Severe | IST |
| 12 | 26 | F | - | 10 | 4,700 (1780) | 8.4 | 14 | 0.9 | Severe | |
| 13 | 36 | F | - | 10 | 800 (550) | 6.9 | 9 | 0.2 | Severe | BMT |
| 14 | 57 | F | - | 15 | 3,700 (1510) | 11.3 | 67 | 0.8 | ||
| 15 | 23 | F | - | 15 | 1400 (20) | 5.7 | 4 | 0.1 | Very severe | PBSCT |
| 16 | 25 | M | - | 20 | 900 (290) | 5.0 | 11 | 0.3 | Severe | IST |
| 17 | 69 | F | HBV | 10 | 3,800 (1700) | 10.0 | 73 | 0.9 | ||
| 18 | 33 | F | - | 10 | 3,200 (1120) | 9.9 | 27 | 0.8 | ||
| 19 | 26 | M | - | 5 | 2,600 (1200) | 15.1 | 86 | 0.8 | ||
| 20 | 18 | F | - | 10 | 2,740 (750) | 5.7 | 23 | 0.8 |
Abbreviations: -, not detected; HBV, hepatitis B virus; a, degree of severity of aplastic anemia (‘severe’ was defined as two of following criteria: neutrophils ≤500×103/μL, platelet ≤20×103/μL, corrected reticulocyte count ≤1% and ‘very severe’ defined as the criteria of ‘severe’ plus neutrophils ≤200×103/μL); IST, immunosuppressive therapy; BMT, bone marrow transplantation; PBSCT, peripheral blood stem cell transplantation.
Table 2.
T cell clonality detected in bone marrow specimens of aplastic anemia patients
Table 3.
Changes of T cell clonality in bone marrow specimens after stem cell transplantation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download