Abstract
Background:
Korean national cancer screening program selected fecal occult blood test (FOBT) as a primary screening method of colorectal carcinoma in adult ≥50 yr old irrespective of symptom. Notice to pre-analytical errors is especially important for the FOBT because examinees collect and submit their specimens to laboratories by themselves. We examined the influences of the fecal storage temperatures, durations and with or without buffer on the FOBT results.
Methods:
Thirty FOBT-positive specimens above 100 ng/mL were used for the study from July to August 2008. Quantitative FOBT was performed with OC-sensors II (Eiken Chemical Co., Japan). Each specimen was divided into 4 groups. Two groups in plastic buffer-free containers were kept either at 4°C or room temperature (25-28°C), respectively. Another two groups in buffer-tubes were also kept either at 4°C or room temperature. Each group was repeatedly examined with same method every 24 hr up to 120 hr.
Results:
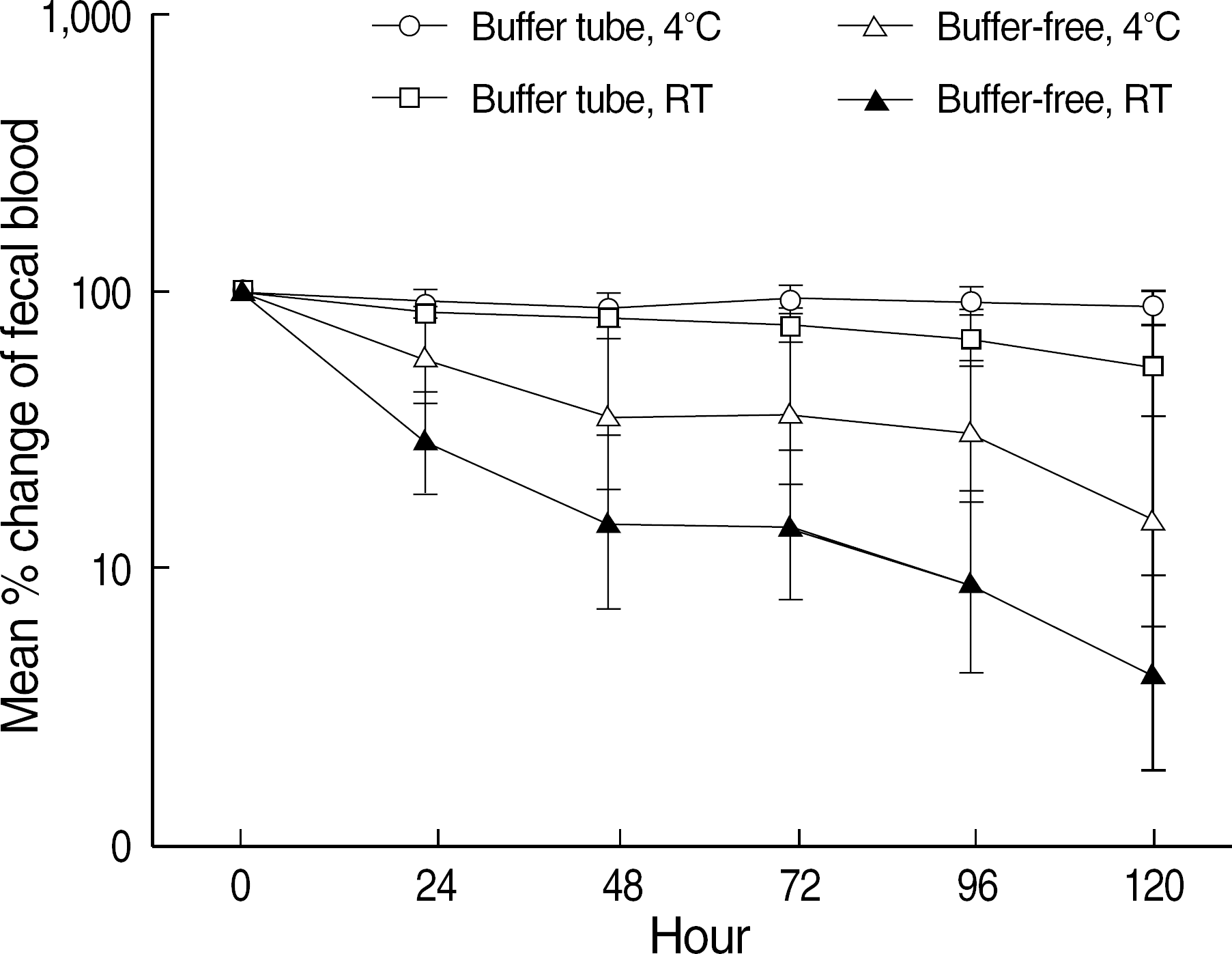
Eleven specimens (36.7%) in buffer-free containers converted to negative results (below the 100 ng/mL) after 24 hr and 17 specimens (56.7%) did after 48 hr at room temperature. Ten specimens (33.3%) in buffer-free containers converted to negative after 48 hr at 4°C. Specimens contained in buffer-tubes showed little change; 3 specimens (10.0%) at room temperature and no specimen at 4°C showed negative conversions after 48 hr.
REFERENCES
1.Korea National Statistical Office. 2007 death and death cause statistics. http://www.nso.go.kr. (Updated on Sep. 2008. (통계청. 2007년사망 및 사망원인통계결과.http://www.nso.go.kr. (2008 년9월 보도자료.).
2.Mandel JS., Bond JH., Church TR., Snover DC., Bradley GM., Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993. 328:1365–71.
3.Mandel JS., Church TR., Bond JH., Ederer F., Geisser MS., Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000. 343:1603–7.

4.Winawer SJ., Flehinger BJ., Schottenfeld D., Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993. 85:1311–8.

5.Kronborg O., Fenger C., Olsen J., Jorgensen OD., Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996. 348:1467–71.

6.Ahlquist DA., Shuber AP. Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clin Chim Acta. 2002. 315:157–68.

7.Cole SR., Young GP. Effect of dietary restriction on participation in faecal occult blood test screening for colorectal cancer. Med J Aust. 2001. 175:195–8.

8.Cole SR., Young GP., Esterman A., Cadd B., Morcom J. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen. 2003. 10:117–22.

9.Ouyang DL., Chen JJ., Getzenberg RH., Schoen RE. Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol. 2005. 100:1393–403.

10.Smith A., Young GP., Cole SR., Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006. 107:2152–9.

11.Young GP., St John DJ., Winawer SJ., Rozen P. Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol. 2002. 97:2499–507.

12.Han WS., Choi YM., Lee DH. Comparison of guaiac method with latex method for the fecal occult blood test. Korean J Clin Pathol. 1996. 16:318–23. (한원선, 최윤미, 이도훈. 변잠혈검사에 대한 guaiac 법과 latex 법의비교. 대한임상병리학회지 1996;16:318-23.).
13.Ministry of Health & Welfare and National Cancer Center. eds. Quality guidelines of colorectal cancer screening. Gyounggi: Ministry of Health & Welfare and National Cancer Center. 2008. :14-6. (보건복지부및국립암센터. 대장암검진질지침. 경기도: 보건복지부및국립암센터,. 2008:14–6.
14.Park EC. Colorectal cancer screening; current problems and future directions. Intestinal Research. 2006. 4(S1):S56–65. (박은철. 대장암조기검진의현황및발전방향. 대한장연구학회 2006;4(부록1):S 56-65.).
15.Malila N., Anttila A., Hakama M. Colorectal cancer screening in Finland: details of the national screening programme implemented in Autumn 2004. J Med Screen. 2005. 12:28–32.

16.Wong BC., Wong WM., Cheung KL., Tong TS., Rozen P., Young GP, et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment Pharmacol Ther. 2003. 18:941–6.

Fig. 1.
Percent change of fecal occult blood test results of 30 positive specimens for 5 days in different storage conditions. Percent value was calculated compared to each of 0 hr result and log transformation was performed. Points indicate mean percent values and vertical lines are 95% confidence intervals for mean values. Abbreviation: RT, room temperature.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download