Abstract
Background:
Real-time PCR for quantification of JAK2 V617F has recently been introduced and used to evaluate the importance of mutant allele burden in both diagnosis and disease progression in myeloproliferative diseases (MPDs). We evaluated the usefulness of JAK2 MutaScreen™ kit that uses a real-time semiquantitative PCR method and has been designed to screen JAK2 V617F mutant allele burden.
Methods:
Forty MPD patients were included in this study. We screened JAK2 V617F and determined the mutant allele burden using JAK2 MutaScreen™ kit. The mutant allele burden was estimated by six-scaled standards of JAK2 V617F mutant allele (2%, 5%, 12.5%, 31%, 50%, and 78%). For evaluation of test performance, an allele-specific PCR (AS-PCR) was carried out in all samples by using Seeplex JAK2 Genotyping kit. We assessed the clinical differences in distinct disease entities of MPDs according to JAK2 V617F mutant allele burden.
Results:
JAK2 V617F mutation was detected in 30 cases, including 10 of 11 cases (91%) of polycythemia vera (PV), 13 of 20 cases (65%) of essential thrombocythemia (ET), and 2 of 3 cases (67%) of chronic idiopathic myelofibrosis (CIMF). The concordance rate between the two tests was 95% (38/40). JAK2 V617F mutant allele burden was greater than 50% in 17 cases, and 10 of them (59%) were PV. In contrast, mutant allele burden was less than 50% in 13 cases and 11 of them (85%) were ET.
REFERENCES
1.James C., Ugo V., Le Couédic JP., Staerk J., Delhommeau F., Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005. 434:1144–8.

2.Baxter EJ., Scott LM., Campbell PJ., East C., Fourouclas N., Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005. 365:1054–61.

3.Levine RL., Wadleigh M., Cools J., Ebert BL., Wernig G., Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005. 7:387–97.

4.Kralovics R., Passamonti F., Buser AS., Teo SS., Tiedt R., Passweg JR, et al. A gain-of-function mutation in myeloproliferative disorders. N Engl J Med. 2005. 352:1779–90.
5.Tefferi A., Thiele J., Orazi A., Kvasnicka HM., Barbui T., Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007. 110:1092–7.

6.Levine RL., Wadleigh M., Cools J., Ebert BL., Wernig G., Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005. 7:387–97.

7.Jones AV., Kreil S., Zoi K., Waghorn K., Curtis C., Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005. 106:2162–8.

8.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951. 6:372–5.
9.Kaushansky K. On the molecular origins of the chronic myeloproliferative disorders: it all makes sense. Blood. 2005. 105:4187–90.

10.Lacout C., Pisani DF., Tulliez M., Gachelin FM., Vainchenker W., Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006. 108:1652–60.

11.Poodt J., Fijnheer R., Walsh IB., Hermans MH. A sensitive and reliable semi-quantitative real-time PCR assay to detect JAK2 V617F in blood. Hematol Oncol. 2006. 24:227–33.
12.Vannucchi AM., Antonioli E., Guglielmelli P., Rambaldi A., Barosi G., Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007. 110:840–6.
13.Wolstencroft EC., Hanlon K., Harries LW., Standen GR., Sternberg A., Ellard S. Development of a quantitative real-time polymerase chain reaction assay for the detection of the JAK2 V617F mutation. J Mol Diagn. 2007. 9:42–6.

14.Ahn JY., Yoo SJ., Bang SM., Park PW., Seo YH., Shin DB, et al. JAK2V617F mutation in Korean patients with essential thrombocythemia. Korean J Lab Med. 2007. 27:77–82. (안정열, 유수진, 방수미, 박필환, 서일혜,신동복 등. 한국인 본태성혈소판혈증 환자에서 JAK2V617F 유전자 변이.대한진단검사의학회지 2007;27:77-82.).
15.Baxter EJ., Scott LM., Campbell PJ., East C., Fourouclas N., Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005. 365:1054–61.

16.Scott LM., Tong W., Levine RL., Scott MA., Beer PA., Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007. 356:459–68.
17.Larsen TS., Pallisgaard N., Moller MB., Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibro—impact on disease phenotype. Eur J Haematol. 2007. 79:508–15.
18.Passamonti F., Rumi E., Pietra D., Della Porta MG., Boveri E., Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006. 107:3676–82.

19.Lippert E., Boissinot M., Kralovics R., Girodon F., Dobo I., Praloran V, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006. 108:1865–7.

20.Vannucchi AM., Pancrazzi A., Bogani C., Antoniolo E., Guglielmelli P. A quantitative assay for JAKV617F mutation in myeloproliferative disorders by ARMS-PCR and capillary electrophoresis. Leukemia. 2006. 20:1055–60.
21.Tefferi A., Lasho TL., Schwager SM., Strand JS., Elliott M., Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006. 106:631–5.

22.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008. 22:23–30.
Fig. 1.
Distribution of JAK2 V617F allele burden determined by JAK2 MutaScreen™ kit in 30 cases of JAK2 V617F positive MPDs. Abbreviations: PV, polycythemia vera; ET, essential thrombocythemia; CIMP, chronic idiopathic myelofibrosis; MPD-U, myeloproliferative disease, unclassifiable.
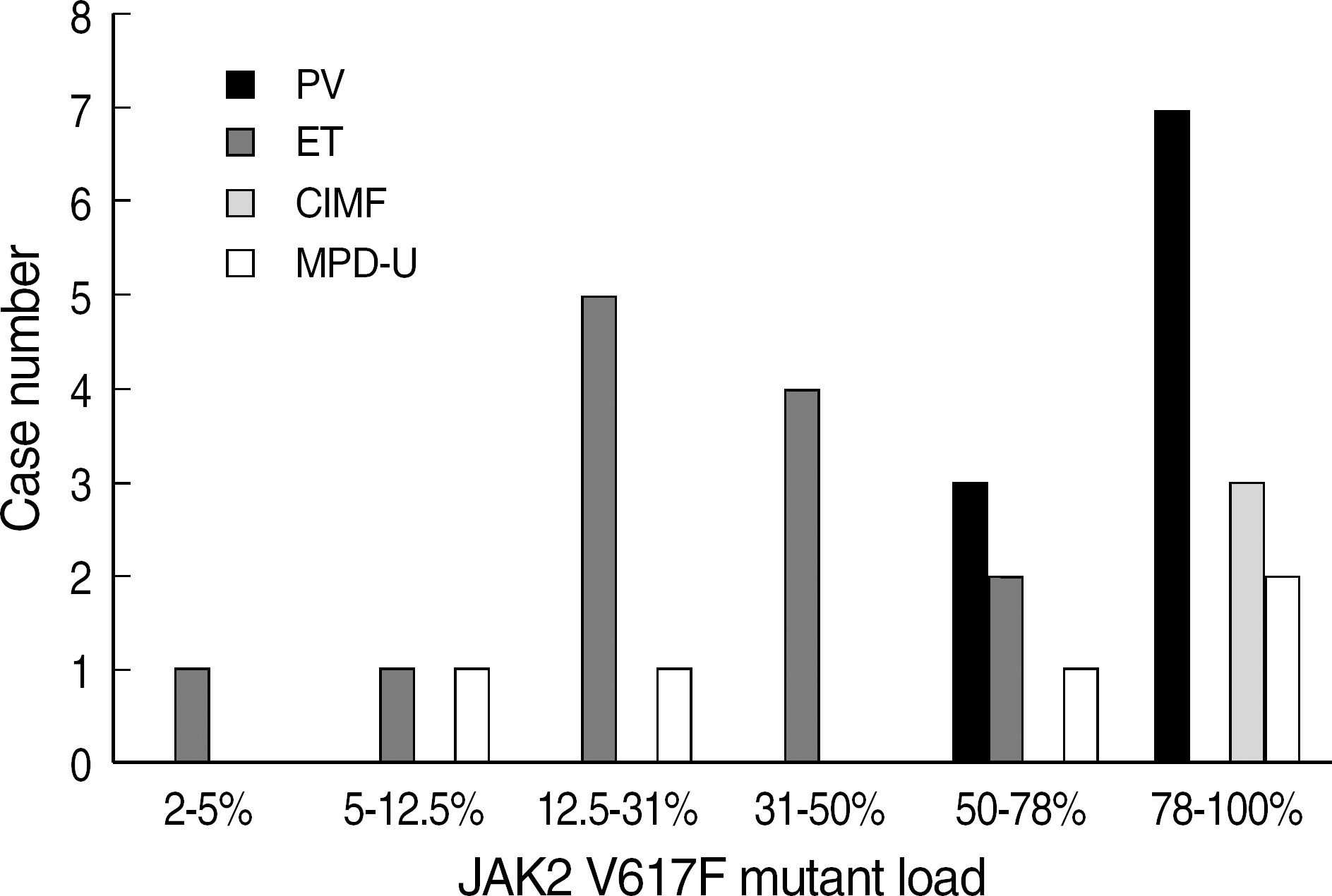
Table 1.
Clinical characteristics of 40 MPDs patients according to JAK2 V617F mutation status determined by JAK2 MutaScreen™ kit
| Characteristics | JAK2 V617F | |
|---|---|---|
| Present (N=30) | Absent (N=10) | |
| Diagnosis | ||
| PV (N=11) | 10 (91%) | 1 (9%) |
| ET (N=20) | 13 (65%) | 7 (35%) |
| CIMF (N=3) | 2 (67%) | 1 (33%) |
| MPD-U (N=5) | 5 (100%) | 0 (0%) |
| MDS/MPD-U (N=1) | 0 (0%) | 1 (100%) |
| Complications∗ | 8 | 7 |
| Abnormal karyotype | 2 | 1 |
| Palpable spleen | 8 | 0 |
| Cytoreductive treatment | 22 | 5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download