Abstract
Background:
Since metallo-β-lactamase (MBL)-producing isolates can hydrolyze carbapenem and also easily transfer the resistance genes to other bacteria, a rapid and accurate detection of MBL has become very important. We evaluated the utility of Mueller Hinton agar (MHA) biplate containing dipicolinic acid (DPA) as a screening method to detect IMP-1 and VIM-2 type MBL-producing isolates.
Methods:
Based on our preliminary tests using various concentrations of DPA, 200 and 300 μg/mL concentration of DPA were chosen for further study. Bacterial lawns were grown on MHA biplate, one half of which contained DPA while the other did not. The inhibition zone around the imipenem (IPM) disk on both sides of this plate was compared. The stability of DPA in the stored DPA-MHA biplate was also evaluated during three months using two MBL- and one non-MBL-producing isolates.
Results:
When the criterion of a ≥7 mm increase of inhibition zone around the IPM disk on the MHA containing DPA compared to MHA without DPA was used, the sensitivities and specificities were 94.7% and 97.6% for 200 μg/mL DPA-MHA biplate, and 98.2% and 97.6% for 300 μg/mL DPA-MHA biplate, respectively. The activity of the DPA in this biplate was stable for three months.
Go to : 
REFERENCES
1.Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980. 289:321–31.
2.Bush K., Jacoby GA., Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995. 39:1211–33.
3.Watanabe M., Iyobe S., Inoue M., Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991. 35:147–51.
4.Lauretti L., Riccio ML., Mazzriol A., Cornaglia G., Amicosante G., Fontana R, et al. Cloning and characterization of blaVIM, a new integronborne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999. 43:1584–90.
5.Lee K., Lee WG., Uh Y., Ha GY., Cho J., Chong Y. VIM-and IMP-type metallo-β-lactamase producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003. 9:868–71.
6.Riccio ML., Franceschini N., Boschi L., Caravelli B., Cornaglia G., Fontana R, et al. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob Agents Chemother. 2000. 44:1229–35.
7.Toleman MA., Simm AM., Murphy TA., Gales AC., Biedenbach DJ., Jones RN, et al. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial programme. J Antimicrob Chemother. 2002. 50:673–9.
8.Castanheira M., Toleman MA., Jones RN., Schmidt FJ., Walsh TR. Molecular characterization of a β-lactamase gene, blaGIM-1, encoding a new subclass of metallo-β-lactamase. Antimicrob Agents Chemother. 2004. 48:4654–61.
9.Lee K., Yum JH., Yong D., Lee HM., Kim HD., Docquier JD, et al. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005. 49:4485–91.
10.Arakawa Y., Shibata N., Shibayama K., Kurokawa H., Yagi T., Fujiwara H, et al. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by thiol compounds. J Clin Microbiol. 2000. 38:40–3.
11.Lee K., Chong Y., Shin HB., Kim YA., Yong D., Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001. 7:88–91.
12.Chu YW., Cheung TK., Ngan JY., Kam KM. EDTA susceptibility leading to false detection of metallo-β-lactamase in Pseudomonas aeruginosa by Etest and an imipenem-EDTA disk method. Int J Antimicrob Agents. 2005. 26:340–1.
13.Kimura S., Ishii Y., Yamaguchi K. Evaluation of dipicolinic acid for detection of IMP- or VIM- type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates. Diagn Microbiol Infect Dis. 2005. 53:241–4.
14.Lee K., Lim YS., Yong D., Yum JH., Chong Y. Evaluation of Hodge test and imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003. 41:4623–9.
15.Siemann S., Brewer D., Clarke AJ., Dmitrienko GI., Lajoie G., Viswanatha T. IMP-1 metallo-β-lactamase: effect of chelators and assessment of metal requirement by electrospray mass spectrometry. Biochim Biophys Acta. 2002. 1571:190–200.
16.Shin KS., Son BR., Hong SB., Kim J. Dipicolinic acid-based disk methods for detection of metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. Diagn Microbiol Infect Dis. 2008. 62:102–5.
17.Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. CLSI document M100-S16, Wayne Clinical and Laboratory Standards Institute. 2006.
18.Shin KS., Han K., Lee J., Hong SB., Son BR., Youn SJ, et al. Imipenem-resistant Achromobacter xylosoxidans carrying blaVIM-2-containing class 1 integron. Diagn Microbiol Infect Dis. 2005. 53:215–20.
19.Kim J., Shin KS. Production of VIM-2 type metallo-β-lactamase in urinary isolates Providencia rettgeri. Korean J Lab Med. 2005. 25:399–405. (김정민 및 신경섭. 요검체에서 분리된 Providencia rettgeri 에서VIM-2형 metallo-β-lactamase 의생성. 대한진단검사의학회지 2005;25:399-405.).
20.Kim IS., Oh WI., Song JH., Lee NY. Screening and identification of metallo-β-lactamase gene in clinical isolates of imipenem-resistant Pseudomonas aeruginosa. Korean J Lab Med. 2004. 24:177–82. (김인숙,오원일, 송재훈, 이남용. 임상 검체에서 분리된 imipenem 내성 녹농균의 metallo-β-lactamase 유전자 선별 및 확인. 대한진단검사의학회지 2004;24:177-82.).
21.Poirel R., Naas T., Nicolas D., Collect L., Bellais S., Cavallo JD, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolates in France. Antimicrob Agents Chemother. 2000. 44:891–7.
22.Yong D., Lee K., Yum JH., Shin HB., Rossolini GM., Chong Y. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002. 40:3798–801.
23.Lee K., Kim MN., Choi TY., Cho SE., Lee S., Whang DH, et al. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009. 33:520–4.
24.Pournaras S., Markogiannakis A., Ikonomidis A., Kondyli L., Bethimouti K., Maniatis AN, et al. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J Antimicrob Chemother. 2006. 57:557–61.
25.Yana JJ., Wub JJ., Tsai SH., Chuang CL. Comparison of the double-disk, combined disk, and Etest methods for detecting metallo-β-lactamases in gram-negative bacilli. Diagn Microbiol Infect Dis. 2004. 49:5–11.
26.Lee K., Yong D., Yum JH., Lim YS., Bolmström A., Qwärnström A, et al. Evaluation of Etest MBL for detection of blaIMP-1 and blaVIM-2 allele-positive clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2005. 43:942–4.
Go to : 
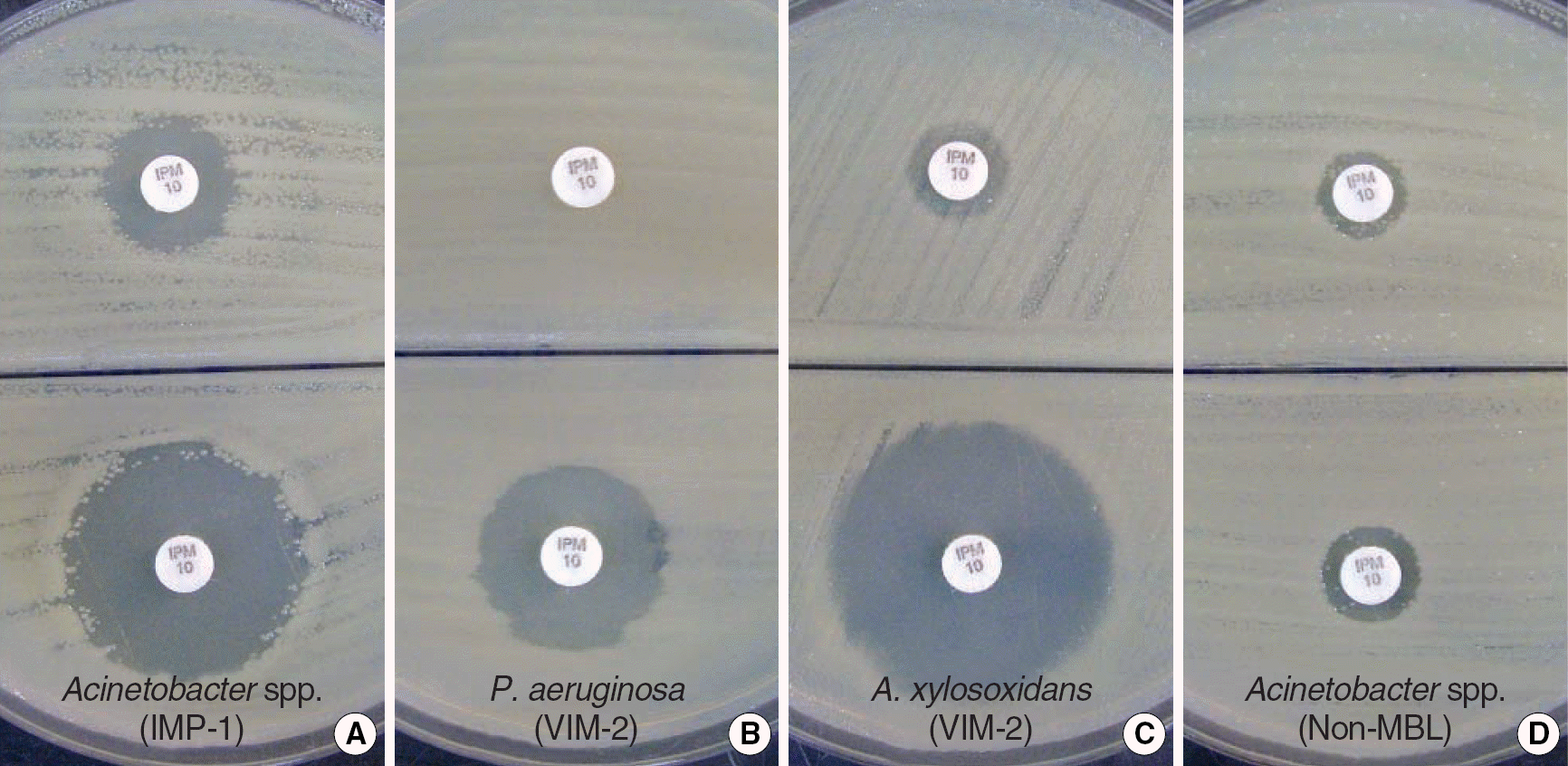 | Fig. 1.DPA-based MHA biplate for the detection of MBL in IPM non-susceptible clinical isolates. (A) IMP-1 type Acinetobacter spp., (B) VIM-2 type Pseudomonas aeruginosa, (C) VIM-2 Achromobacter xylosoxidans, and (D) non-MBL producing Acinetobacter spp. The lower part of each biplates contained 300 μg/mL DPA. MBL producing isolates (A-C) showed increased inhibition zones (≥7 mm) around IPM disks in the presence of DPA as compared to disks that lacked DPA. The non-MBL-producing isolate (D) showed no significant difference in inhibition zone size.
Abbreviations: IPM, imipenem; MHA, Mueller Hinton agar; DPA, dipicolinic acid.
|
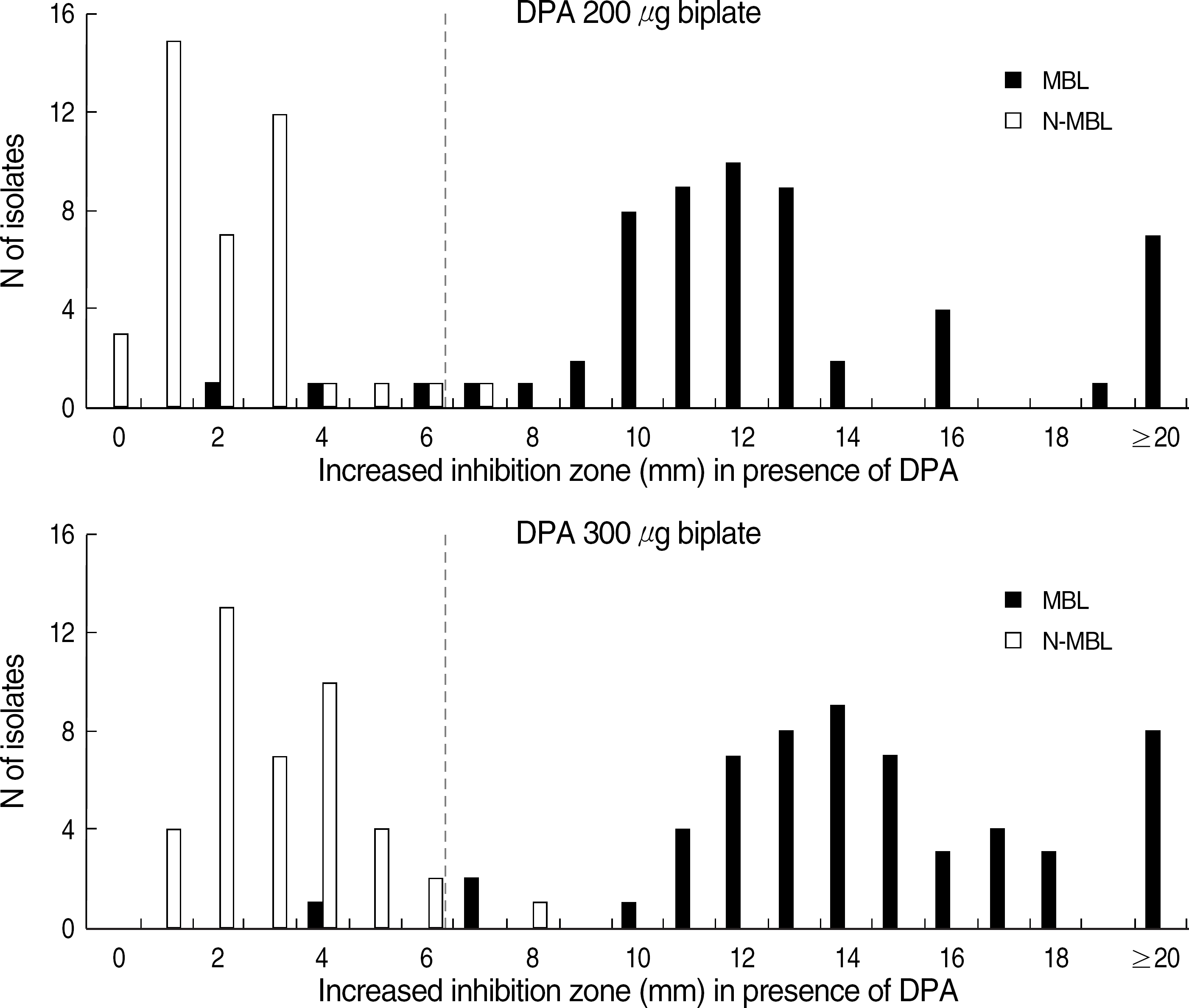 | Fig. 2.Increase of inhibition zone size around the imipenem disk in MHA with DPA as compared with inhibition zones produced by imipenem disk in MHA without DPA for 57 MBL positive and 41 MBL negative clinical isolates.
Abbreviations: DPA, dipicolinic acid; MHA, Mueller Hinton agar; MBL, metallo-β-lactamase; N-MBL, non-MBL.
|
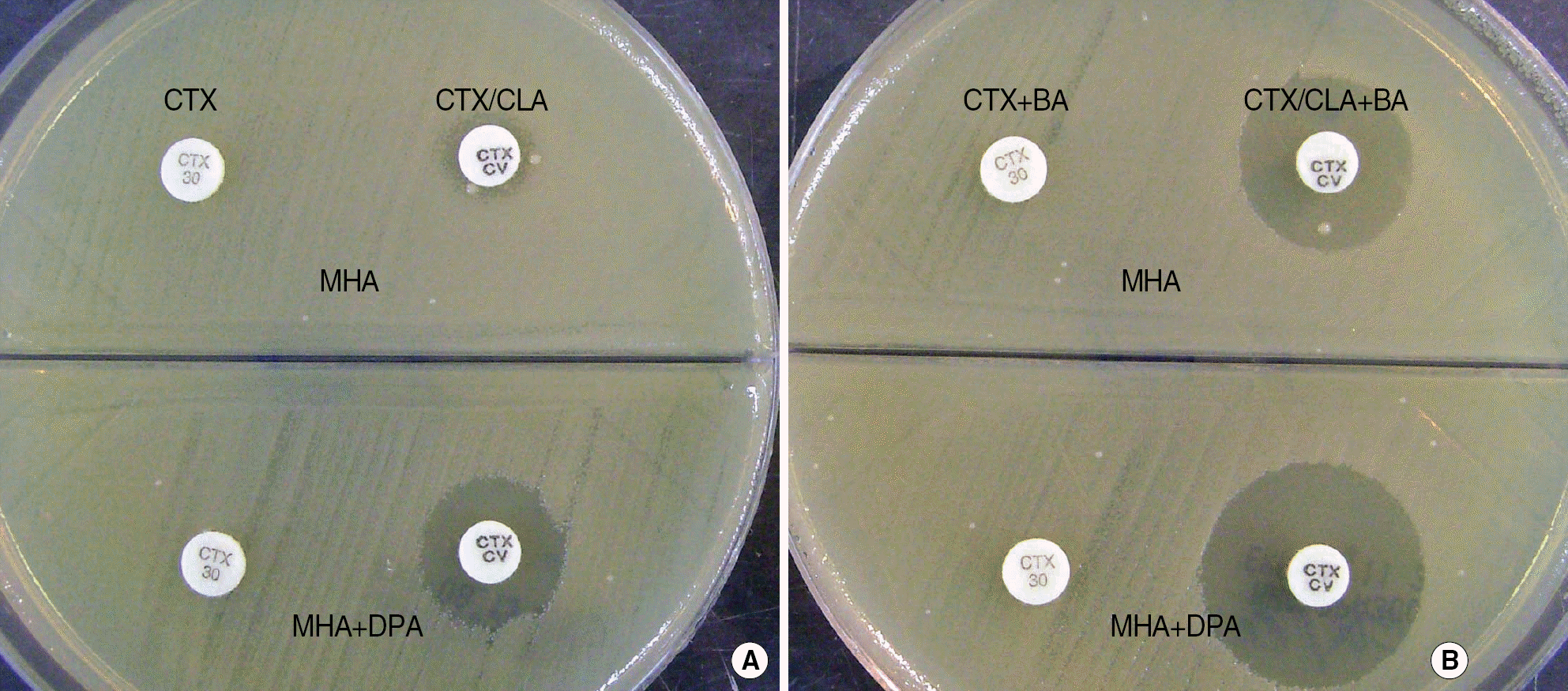 | Fig. 3.DPA-based MHA biplate for the detection of three type β-lactamase from Providencia rettgerii carrying ESBL, AmpC β-lactamase and MBL. When boronic acid (BA) was add to cefotaxime (CTX) and cefotaxime/clavulanic acid (CTX/CLA) disk, a ≥5 mm increase of inhibition zone around CTX/CLA disk in comparison to CTX disk suggested the co-production of ESBL and AmpC β-lactamase (A→B), and the increase of inhibition zone around CTX/CLA disks on DPA-containing MHA relative to MHA at both A and B suggested the concurrent production of MBL (upper→lower).
Abbreviations: DPA, dipicolinic acid; MHA, Mueller Hinton agar; ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase.
|
Table 1.
Results of dipicolinic acid-containing Mueller Hinton agar biplate for detection of metallo-β-lactamase in imipenem non-susceptible gram-negative bacilli
| Species and allele gene of MBL genes (N of isolates) | DPA 200-MHA biplate | DPA 300-MHA biplate | ||
|---|---|---|---|---|
| Positive† | Negative | Positive | Negative | |
| Pseudomonas aeruginosa (46) | ||||
| IMP-1 type MBL (8) | 8 | 0 | 8 | 0 |
| VIM-2 type MBL (17) | 17 | 0 | 17 | 0 |
| N-MBL (21) | 1 | 20 | 1 | 20 |
| Acinetobacter spp. (45) | ||||
| IMP-1 type MBL (13) | 12 | 1 | 13 | 0 |
| VIM-2 type MBL (12) | 11 | 1‡ | 11 | 1‡ |
| N-MBL (20) | 0 | 20 | 0 | 20 |
| Other isolates∗ (7) | ||||
| VIM-2 type MBL (7) | 6 | 1 | 7 | 0 |
| Total isolates (98) | ||||
| MBL (57) | 54 | 3 | 56 | 1‡ |
| N-MBL (41) | 1 | 40 | 1 | 40 |
| Sensitivity (%) | 94.7 | 98.2 | ||
| Specificity (%) | 97.6 | 97.6 | ||
Table 2.
Stability of dipicolinic acid activity in DPA 300-Mueller Hinton agar biplate during 12 weeks
| Inhibition zone around IPM 10 μg disk (mm) | ||||||
|---|---|---|---|---|---|---|
| Storage (weeks) | IMP-1 Acinetobacter spp. | VIM-2 P. aeruginosa | P. aeruginosa ATCC 27853 | |||
| MHA | DPA 300 μg | MHA | DPA 300 μg | MHA | DPA 300 μg | |
| Immediate | 13 | 28 (15)∗ | 7 | 32 (25) | 23 | 27 (4) |
| 1 | 13 | 28 (15) | 9 | 33 (24) | 23 | 26 (3) |
| 2 | 14 | 28 (14) | 9 | 33 (24) | 23 | 27 (4) |
| 4 | 15 | 28 (13) | 9 | 34 (25) | 25 | 29 (4) |
| 6 | 15 | 28 (13) | 9 | 34 (25) | 23 | 26 (3) |
| 8 | 15 | 28 (13) | 9 | 34 (25) | 25 | 28 (3) |
| 10 | 14 | 28 (14) | 8 | 32 (24) | 25 | 28 (3) |
| 12 | 14 | 28 (14) | 9 | 33 (24) | 24 | 27 (3) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download