Abstract
Background
Human papillomavirus (HPV) prophylactic vaccines, bivalent types for HPV-16/18 with 70% prophylactic expectation, have been developed based on the genotypes found prevalent in the western countries, but little is known for those in Korea. Using a DNA chip test, we evaluated the clinical efficacy of HPV genotype based on cervical abnormalities.
Methods
As the initial diagnostic tests, HPV DNA chip tests and Papanicolaou smear (PAP) were used for 1,211 subjects. Cervical colposcopy directed biopsies were performed for 626 among the 1,211 subjects within one month.
Results
The most frequently found genotypes in all HPV-positive specimens (n=445) were HPV-16 (22.0%), 58 (13.9%), 52 (11.0%), 51 (9.0%), 56 (8.5%), and 18 (7.2%). HPV prevalence was significantly higher in specimens where PAP and biopsy results were closer to malignancy. The HPV genotype distribution of the histologically confirmed cervical high-grade squamous intraepithelial lesions (HSIL) or carcinoma cases showed HPV-16, 58, 52, 18, and 33, in descending order. The HPV DNA chip sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the detection of cervical HSIL or carcinoma were 76.9%, 70.1%, 72.1%, and 75.8%, respectively, Of these, the sensitivity and NPV were higher than those of PAP. PPV and NPV of HPV-16 were 90.5% and 60.7%, respectively, being the highest among the genotypes.
Conclusions
We confirmed that HPV-16 genotype was also very important for the diagnosis of HSIL and cervical carcinoma in Korea. However, contrary to the findings in the western countries, the prevalence of HPV-58 was higher than that of HPV-18. Moreover, as the other HPV genotype reports were rare in Korea, further studies are required with the HPV DNA chip test before the nationwide adoption of the vaccines.
REFERENCES
1.Bosch FX., Manos MM., Munoz N., Sherman M., Jansen AM., Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995. 87:796–802.
2.Clifford GM., Smith JS., Plummer M., Munoz N., Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a metaanalysis. Br J Cancer. 2003. 88:63–73.

3.Hwang HS., Park M., Lee SY., Kwon KH., Pang MG. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004. 13:2153–6.
4.An HJ., Cho NH., Lee SY., Kim IH., Lee C., Kim SJ, et al. Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer. 2003. 97:1672–80.

5.Cho NH., An HJ., Jeong JK., Kang S., Kim JW., Kim YT, et al. Geno-typing of 22 human papillomavirus types by DNA chip in Korean women: comparison with cytologic diagnosis. Am J Obstet Gynecol. 2003. 188:56–62.

6.Kim SR., Song SY., Kim DS., Lee JW., Park CS., Bae DS, et al. Evaluation of self-collected pad sampling for the detection of HPV in cervicovaginal secretion. Korean J Pathol. 2004. 38:258–64.
7.Solomon D., Davey D., Kurman R., Moriarty A., O'Connor D., Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002. 287:2114–9.

8.Gynecologic Oncology Committee of the Korean Society of Obstetrics and Gynecology. Annual report of gynecologic cancer registry program in Korean for 2002 (Jan. 1st, 2002-Dec. 31st, 2002). Korean J Obstet Gynecol. 2004. 47:1029–70. (대한산부인과학회 부인종양위원회. 한국 부인암 등록사업 조사보고서(2002.1.1-2002.12.31). 대한산부인과학회지 2004;47: 1029-70.).
9.Nanda K., McCrory DC., Myers ER., Bastian LA., Hasselblad V., Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000. 132:810–9.

10.Sasieni PD., Cuzick J., Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer. 1996. 73:1001–5.
11.Jung WW., Chun T., Sul D., Hwang KW., Kang HS., Lee DJ, et al. Strategies against human papillomavirus infection and cervical cancer. J Microbiol. 2004. 42:255–66.
12.Franco EL., Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005. 23:2388–94.

13.Adam E., Berkova Z., Daxnerova Z., Icenogle J., Reeves WC., Kaufman RH. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am J Obstet Gynecol. 2001. 182:257–64.

14.Burk RD., Kelly P., Feldman J., Bromberg J., Vermund SH., DeHovitz JA, et al. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex Transm Dis. 1996. 23:333–41.

15.Kulasingam SL., Hughes JP., Kiviat NB., Mao C., Weiss NS., Kuypers JM, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA. 2002. 288:1749–57.
16.de Cremoux P., Coste J., Sastre-Garau X., Thioux M., Bouillac C., Labbe S, et al. Efficiency of the hybrid capture 2 HPV DNA test in cervical cancer screening. A study by the French Society of Clinical Cytology. Am J Clin Pathol. 2003. 120:492–9.
17.Franco EL., Duarte-Franco E., Ferenczy A. Cervical cancer: epidemiology, prevention, and the role of human papillomavirus infection. CMAJ. 2001. 164:1017–25.
18.Solomon D., Schiffman M., Tarone B. ALTS study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001. 93:293–9.

19.Kim JJ., Wright TC., Goldie SJ. Cost-effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst. 2005. 97:888–95.

20.Goldie SJ., Kim JJ., Wright TC. Cost-effeciveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol. 2004. 103:619–31.
21.Yitalo N., Sorensen P., Josefsson AM., Magnusson PK., Andersen PK., Ponten J, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000. 355:2179–80.
Fig. 1.
Percent of patients infected with individual human papillomavirus (HPV) genotypes among age groups. Each type included single and multiple infections.
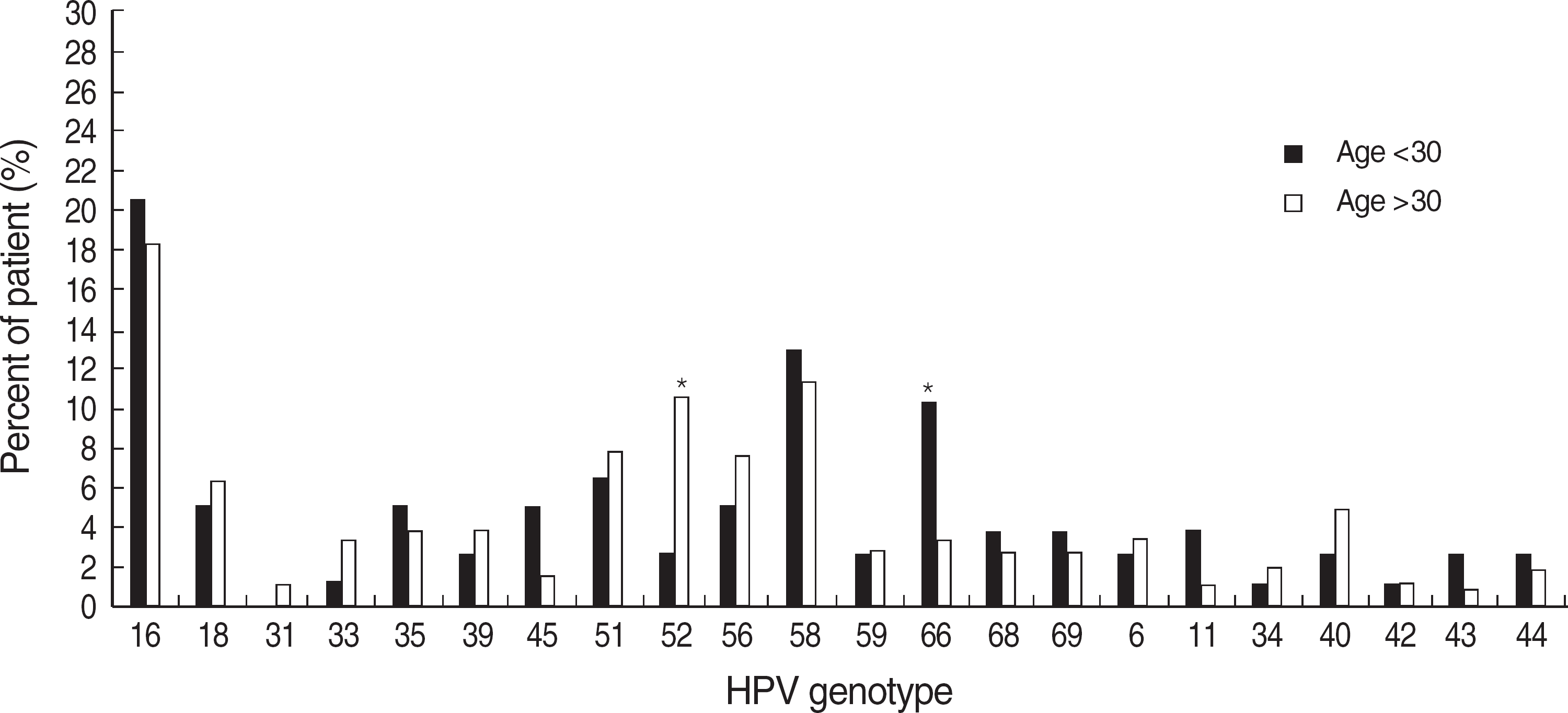
Fig. 2.
Distribution of percent of patients infected with the individual high-risk human papillomavirus (HPV) genotypes. Each type included single and multiple infections. HPV-16 was the most prevalent type in HPV-infected women (chi-square test: P<0.001). LSIL, low-grade squamous intraepithelial lesion.
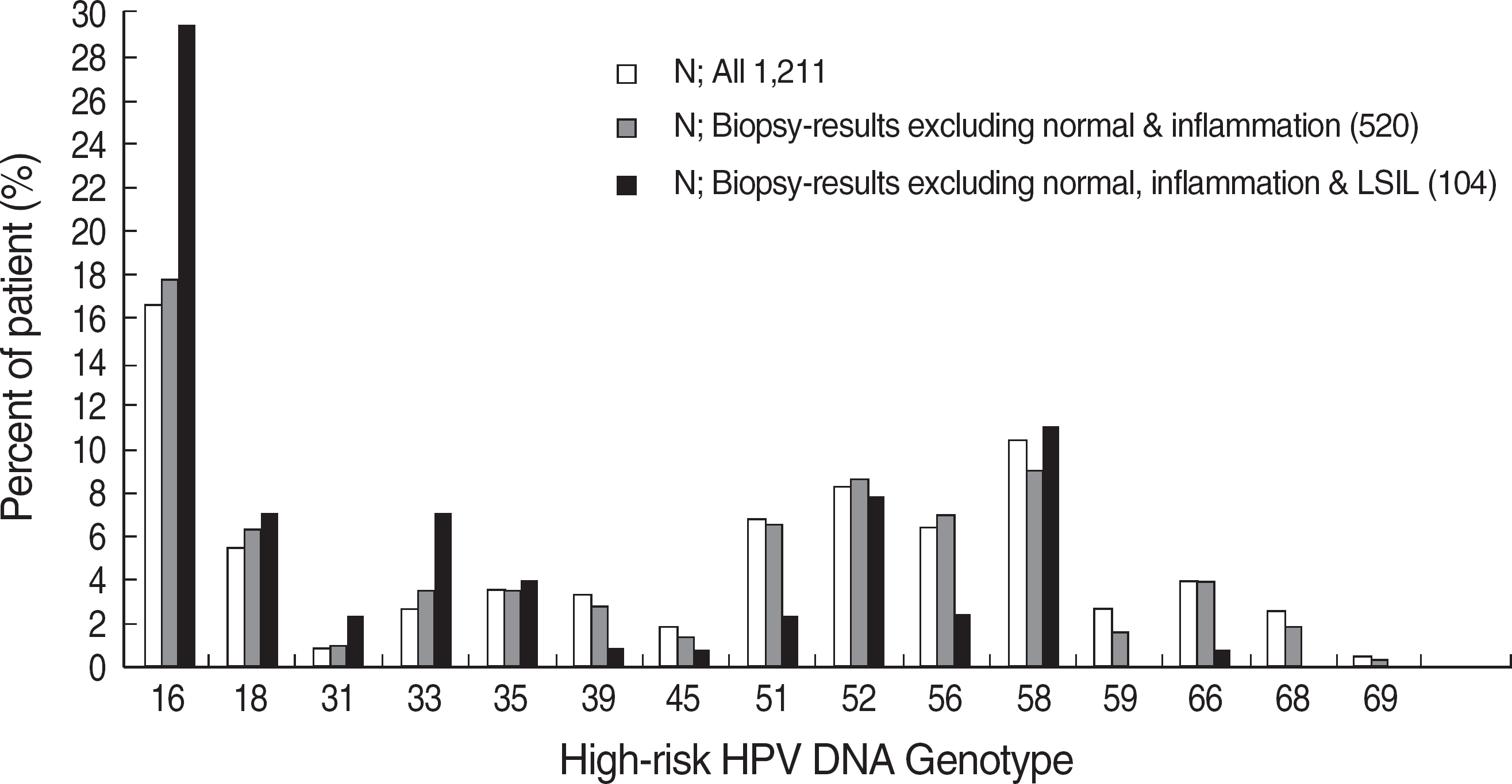
Table 1.
Prevalence of human papillomavirus (high risk groups) according to the results of Papanicolaou smear and biopsy
Table 2.
The distribution of human papillomavirus (HPV) high risk genotypes by HPV DNA chip according to cervical cytology
Table 3.
The distribution of human papillomavirus (HPV) high risk genotypes by HPV DNA chip according to cervical histology
Table 4.
Comparison of test performances of Papanicolaou smear and human papillomavirus testing by DNA chip for the detection of histologically confirmed high-grade squamous intraepithelial lesion or the worse cases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download