Abstract
Background
The presence of rheumatoid factor (RF) is one of the classification criteria of the American College of Rheumatology (ACR) for rheumatoid arthritis (RA), but it has a limitation of low specificity. We compared the diagnostic utility of anti-cyclic citrullinated peptide (CCP) antibodies analyzed by an automated immunoassay system with that measured by a 96 well plate ELISA method.
Methods
The RF and anti-CCP antibodies were determined in 172 serum samples: 52 RA patients, 73 disease controls (systemic lupus, Sjogren's syndrome, palindromic rheumatism), and 47 healthy controls. Anti-CCP antibodies were measured by DIASTAT 96 well plate ELISA method (Axis-Shield Diagnostics, UK) and AxSYM automated microparticle enzyme immunoassay system (Abbott Laboratories, USA). RF was assayed by latex immunoturbidimetry (Toshiba 200 FR, Japan). The diagnostic performances of these tests were compared using a ROC curve analysis, and linearity and precision analysis of AxSYM anti-CCP was carried out.
Results
The sensitivities of RF, DIASTAT anti-CCP, and AxSYM anti-CCP were 78.8%, 84.6%, and 82.7%, respectively and the specificities were 72.5%, 88.3%, and 88.3%, respectively. On ROC curve analysis, the area under the curve was 0.924 for AxSYM anti-CCP, 0.886 for DIASTAT anti-CCP, and 0.847 for RF. AxSYM anti-CCP showed a good linearity, and within-run and total-run precision.
Go to : 
REFERENCES
1.Kasper DL, Fauci AS, editors. eds. Harrison's principles of internal medicine. 16th ed.New York: McGraw-Hill;2004. p. 1968–76.
2.Boers M., Verhoeven AC., Markusse HM., van de Laar MA., Westhovens R., van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997. 350:309–18.

3.Anderson JJ., Wells G., Verhoeven AC., Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000. 43:22–9.

4.Steiner G., Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002. 4(S):S1–5.
5.Arnett FC., Edworthy SM., Bloch DA., McShane DJ., Fries JF., Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31:315–24.

6.Schellekens GA., Visser H., de Jong BA., van den Hoogen FH., Hazes JM., Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000. 43:155–63.

7.van Boekel MA., Vossenaar ER., van den Hoogen FH., van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2002. 4:87–93.
8.Nishimura K., Sugiyama D., Kogata Y., Tsuji G., Nakazawa T., Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007. 146:797–808.

9.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. Document EP6-A. Wayne PA: Clinical and Laboratory Standards Institute;2003.
10.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline-2nd edi. Document EP5-A2. Wayne PA: Clinical and Laboratory Standards Institute;2004.
12.Avouac J., Gossec L., Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006. 65:845–51.

13.Nielen MM., van Schaardenburg D., Reesink HW., van de Stadt RJ., van der Horst-Bruinsma IE., de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004. 50:380–6.

14.van Oosterhout M., Bajema I., Levarht EW., Toes RE., Huizinga TW., van Laar JM. Differences in synovial tissue infiltrates between anti-cyclic citrullinated peptide-positive rheumatoid arthritis and anti-cyclic citrullinated peptide-negative rheumatoid arthritis. Arthritis Rheum. 2008. 58:53–60.

15.Liao KP., Batra KL., Chibnik L., Schur PH., Costenbader KH. Anti-cyclic citrullinated peptide revised criteria for the classification of rheumatoid arthritis. Ann Rheum Dis. 2008. 67:1557–61.

16.Lutteri L., Malaise M., Chapelle JP. Comparison of second- and third-generation anti-cyclic citrullinated peptide antibodies assays for detecting rheumatoid arthritis. Clin Chim Acta. 2007. 386:76–81.

17.Coenen D., Verschueren P., Westhovens R., Bossuyt X. Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis. Clin Chem. 2007. 53:498–504.

18.Cho SY., Kang SY., Lee HJ., Lee WI. A comparative evaluation of the diagnostic value of anti-cyclic citrullinated peptide and rheumatoid factor in rheumatoid arthritis. Korean J Lab Med. 2008. 28:39–45. (조선영, 강소영, 이희주, 이우인. 류마티스 관절염 진단에 있어서 항-Cyclic Citrullinated Peptide 항체와류마티스인자의진단적가치비교평가. 대한진단검사의학회지 2008;28: 39-45.).

19.Salvador G., Gomez A., Vinas O., Ercilla G., Canete JD., Munoz-Gomez J, et al. Prevalence and clinical significance of anti-cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. An abortive form of rheumatoid arthritis? Rheumatology. 2003. 42:972–5.
20.Russell AS., Devani A., Maksymowych WP. The role of anti-cyclic citrullinated peptide antibodies in predicting progression of palindromic rheumatism to rheumatoid arthritis. J Rheumatol. 2006. 33:1240–2.
21.Chan MT., Owen P., Dunphy J., Cox B., Carmichael C., Korendowych E, et al. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol. 2008. 35:77–83.
Go to : 
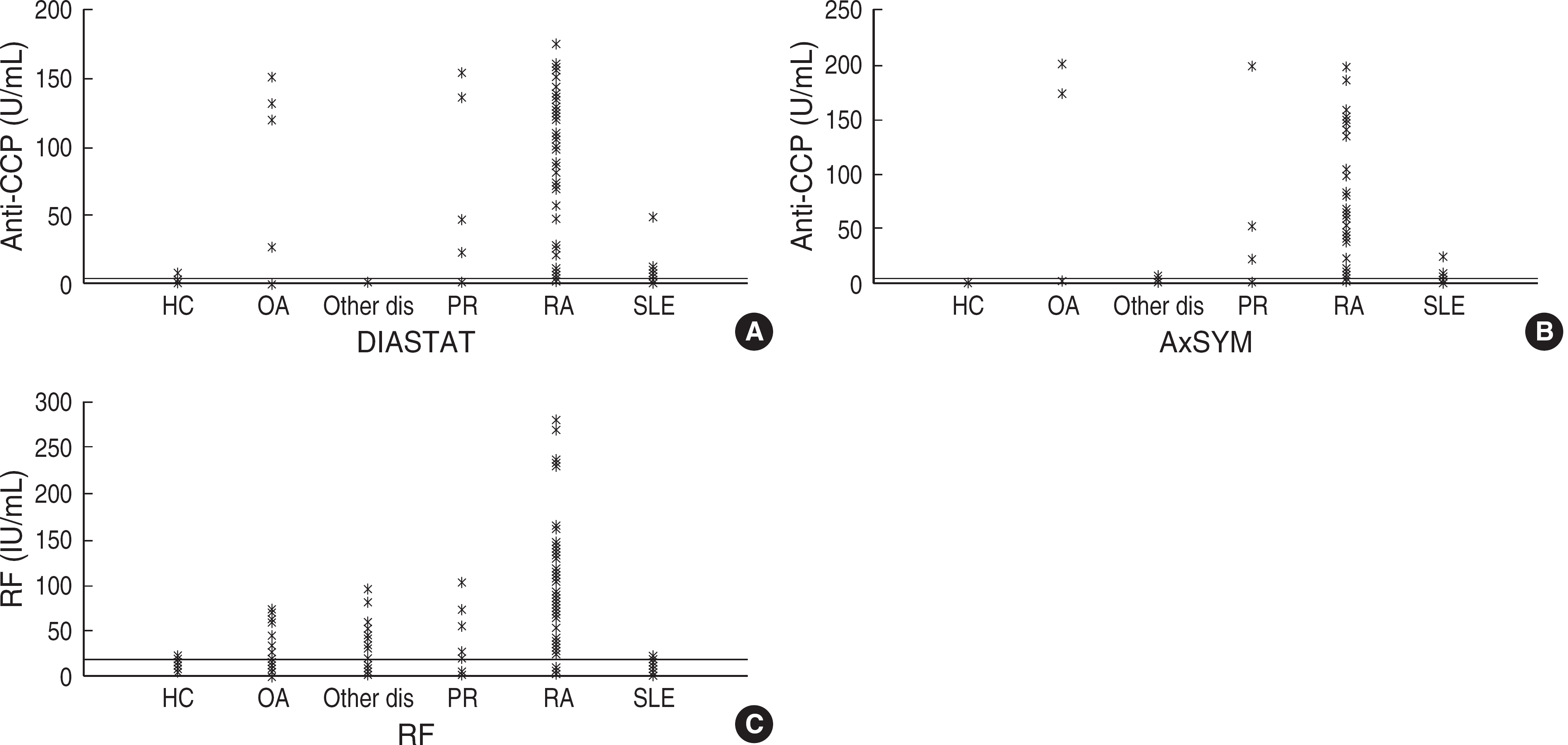 | Fig. 1.Distribution of the test results according to the concentrations of anti-CCP antibodies and RF for different groups of patients. Line: cut-off values ([A] DIASTAT, 5 U/mL; [B] AxSYM, 5 U/mL; [C] RF, 18 IU/mL). Abbreviations: CCP, cyclic citrullinated peptide; RF, rheumatoid factor; HC, healthy control; OA, osteoarthritis; PR, palindromic rheumatism; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus. |
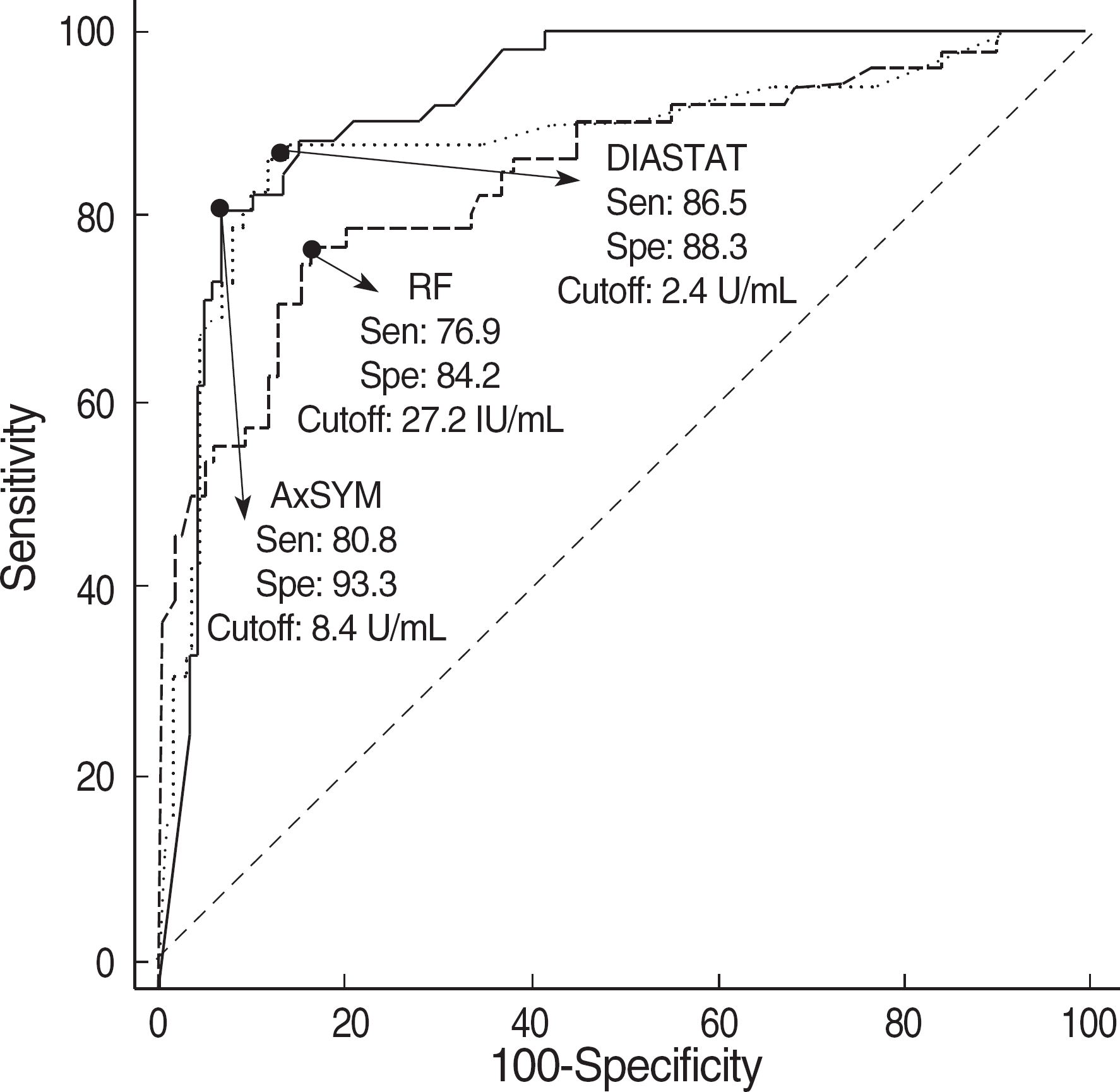 | Fig. 2.The receiver operator characteristics (ROC) curves of Dia-STAT anti-CCP (AUC, 0.888), AxSYM anti-CCP (AUC, 0.923) and RF (AUC, 0.847) in RA patients and control group. There was no significant difference among the AUCs. Abbreviations: Sen, sensitivity; Spe, Specificity; CCP, cyclic citrullinated peptide; RF, rheumatoid factor; RA, rheumatoid arthritis; AUC, area under the curve. |
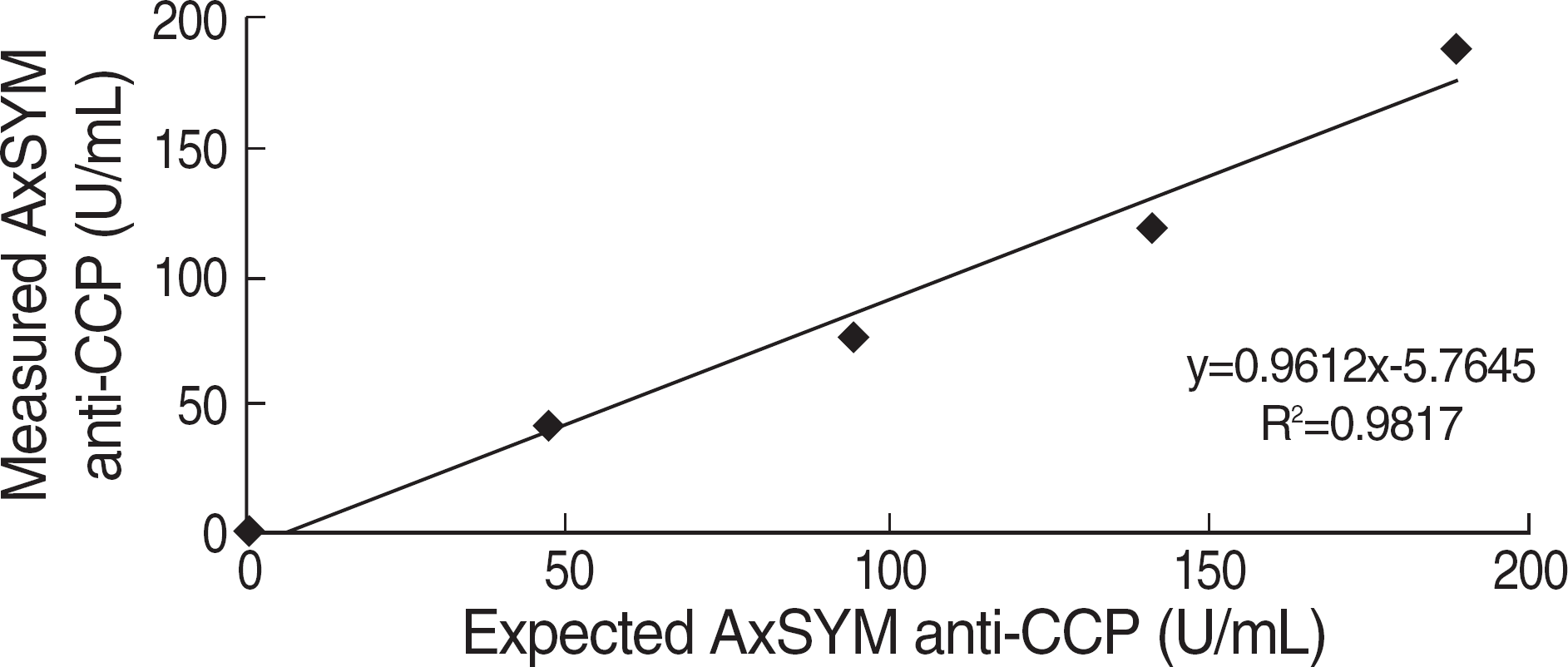 | Fig. 3.Linearity of AxSYM anti-CCP antibody test. Abbreviation: CCP, cyclic citrullinated peptide. Abbreviations: Sen, sensitivity; Spe, Specificity; CCP, cyclic citrullinated peptide; RF, rheumatoid factor; RA, rheumatoid arthritis; AUC, area under the curve. |
Table 1.
The characteristics of rheumatoid arthritis patients and control subjects
| N of subjects | Age (Mean±SD) | M/F | |
|---|---|---|---|
| RA | 52 | 50.2±12.5 | 9/43 |
| Control diseased healthy | 73 | 45.7±14.5 | 21/52 |
| 47 | 45.5±9.8 | 28/19 |
Table 2.
Distribution of positivities of two anti-CCP tests
Table 3.
Comparison of sensitivities and specificities of DIASTAT anti-CCP, AxSYM anti-CCP and RF tests in RA patients and control group using the manufacturer's cut-off values and ROC curves derived cut-off values




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download