Abstract
Background
Despite its unsatisfactory specificity, rheumatoid factor (RF) is the only serologic marker included in the diagnostic criteria of the American College of Rheumatology (ACR) for rheumatoid arthritis. Recently, the diagnostic value of anti-cyclic citrullinated peptide (CCP) antibodies has been emphasized in rheumatoid arthritis (RA) due to its high specificity. To evaluate the second generation of anti-CCP antibodies as a diagnostic marker, we evaluated anti-CCP test in 163 individuals.
Methods
The study population was divided into the following four groups: RA group (n=18), other disease group with arthritic symptoms (n=44), other disease group without arthritic symptoms (n=45), and healthy group (n=56). Anti-CCP was measured by an ELISA analyzer (Coda, Bio-Rad, USA) with Immunoscan RA (Euro-Diagnostica, Malmo, Sweden) and RF was measured by an automated chemistry analyzer (Toshiba, Japan) with RF-LATEX X1 (Denka Seiken, Japan).
Results
The sensitivity of anti-CCP and RF was 72.2% and 100%, respectively, and the respective figures for the specificity were 96.6% and 73%. On each ROC curve, the area under the curve was 0.867 for anti-CCP and 0.959 for RF. In other disease groups, most of the false positive cases of RF were found in the patients with hyperlipidemia or HBV carriage. However, anti-CCP was not detected in any of the patients with these two conditions. False positive rates of RF in the three control groups were 34.1% in other disease group with arthritic symptoms, 48.9% in the other disease group without arthritic symptoms, and 3.6% in healthy group. The respective figures for anti-CCP were 6.8%, 2.2%, and 1.8%.
Conclusions
The specificity of anti-CCP antibodies was higher than that of RF for discriminating RA from other diseases, especially in the patients with hyperlipidemia or HBV carriage. With its high specificity, anti-CCP antibodies can play an additive role in establishing the diagnosis of RA in patients with RF positivity.
Go to : 
REFERENCES
1.Kasper DL, Fauci AS, editors. Harrison's principles of internal medicine. 16th ed.New York: McGraw-Hill;2004. p. 1968–76.
2.Bombardieri M., Alessandri C., Labbadia G., Iannuccelli C., Carlucci F., Riccieri V, et al. Role of anti-cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection-associated polyarticular involvement. Arthritis Res Ther. 2004. 6:R137–41.
3.Goldbach-Mansky R., Lee J., McCoy A., Hoxworth J., Yarboro C., Smolen JS, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000. 2:236–43.

4.Choi SW., Lim MK., Shin DH., Lim CH., Shim SC. Diagnostic performance of the anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis. Korean J Lab Med. 2003. 23:132–8. (최석우, 임미경, 신동혁, 임춘화, 심승철. 류마티스 관절염 환자에서 anti-cyclic citrullinated peptide antibodies 검사의 진단적 유용성. 대한진단검사의학회지 2003;23: 132-8.).
5.Schellekens GA., Visser H., de Jong BA., van den Hoogen FH., Hazes JM., Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000. 43:155–63.

6.Vallbracht I., Helmke K. Additional diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Autoimmun Rev. 2005. 4:389–94.

7.Boers M., Verhoeven AC., Markusse HM., van de Laar MA., Westhovens R., van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulpha-salazine alone in early rheumatoid arthritis. Lancet. 1997. 350:309–18.

8.Rose NR, Hamilton RG, editors. Manual of clinical laboratory immunology. 6th ed.Washington: ASM press;2002. p. 659–64.
9.Choi SW., Lim MK., Shin DH., Park JJ., Shim SC. Diagnostic performances of anti-cyclic citrullinated peptides antibody and antifilag-grin antibody in Korean patients with rheumatoid arthritis. J Korean Med Sci. 2005. 20:473–8.

10.Kang HJ., Seo YI., Lee YK., Cho HC. Diagnostic usefulness of the anti-cyclic citrullinated peptide antibodies for rheumatoid arthritis. J Korean Rheum Assoc. 2003. 10:117–25. (강희정, 서영일, 이영경, 조현찬. 류마티스 관절염에서 항 cyclic citrullinated peptide 항체의 진단적 유용성. 대한류마티스학회지 2003;10: 117-25.).
11.Kim KH. Diagnostic role of the anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis. Korean J Lab Med. 2003. 23:405–10. (검사의 진단적 유용성. 대한진단검사의학회지 2003;23: 405-10.).
12.Kaplan LA, Pesce A, editors. Clinical chemistry: theory, analysis, correlation. 3rd ed.St.Louis: Mosby;1996. p. 427–8.
13.Lee DM., Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003. 62:870–4.

14.Vallbracht I., Rieber J., Oppermann M., Forger F., Siebert U., Helmke K. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis. 2004. 63:1079–84.

15.Nielen MM., van der Horst AR., van Schaardenburg D., van der Horst-Bruinsma IE., van de Stadt RJ., Aarden L, et al. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann Rheum Dis. 2005. 64:1199–204.
16.Avouac J., Gossec L., Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006. 65:845–51.

Go to : 
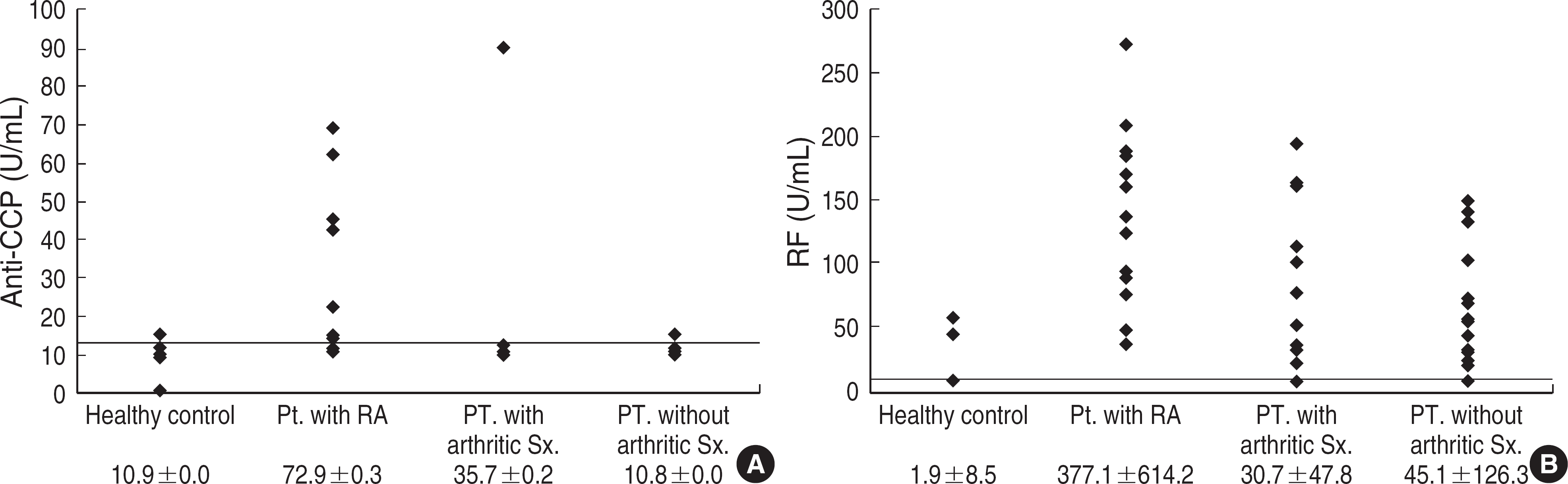 | Fig. 1.The levels of anti-CCP and RF in the serum of patients and healthy contols. (A) anti-CCP test, (B) RF test. Line: Cut-off values (RF, 20 IU/mL; anti-CCP, 12.7 U/mL). |
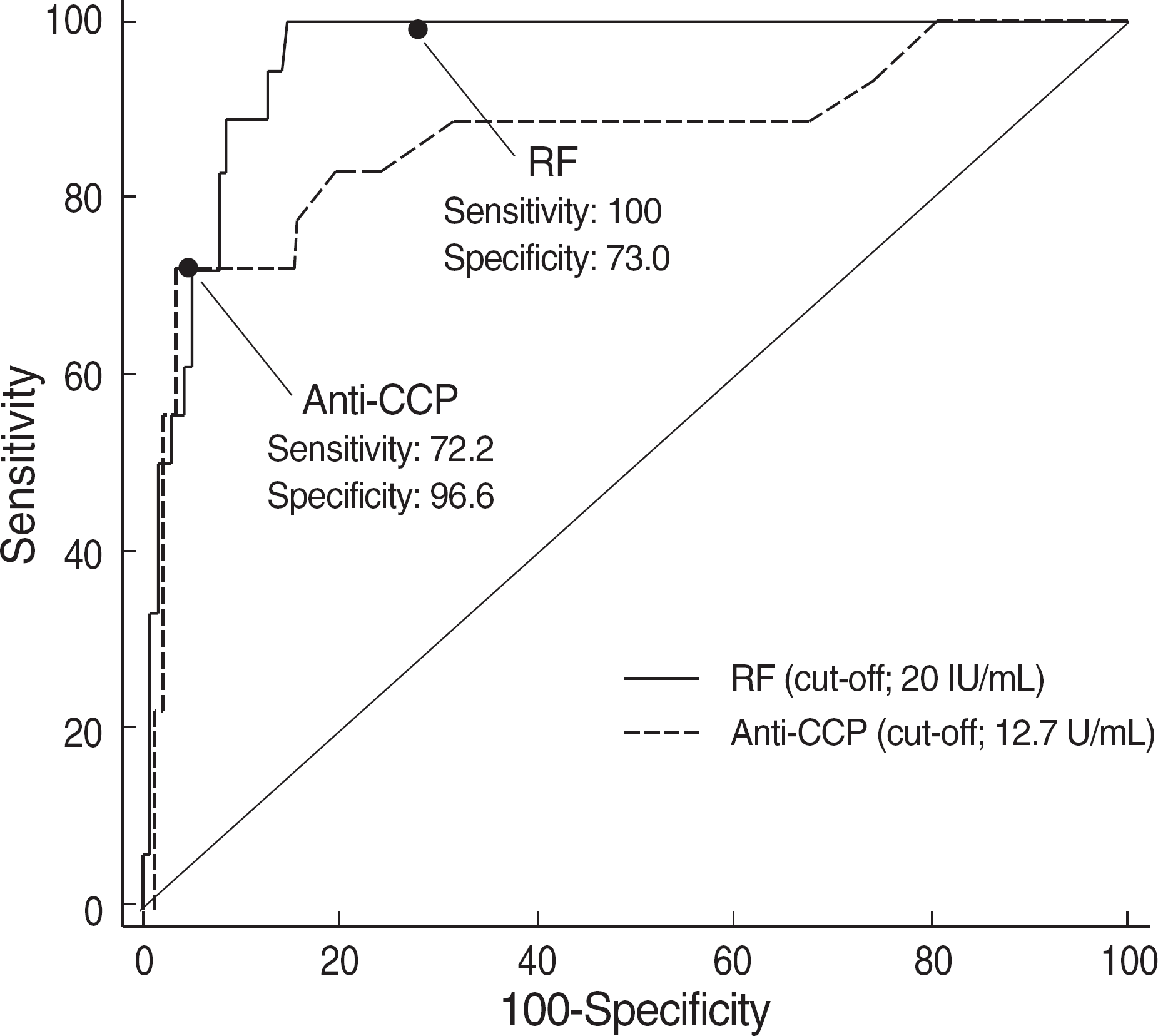 | Fig. 2.The receiver operator characteristics (ROC) curve of anti-CCP and RF.
Areas under curves of RF and Anti-CCP were not significantly different (P=0.134).
|
Table 1.
The distribution of positivity of anti-CCP and RF in RA group and non-RA group
| RA group (n=18) | Non-RA group (n=145) | P∗ value | |||
|---|---|---|---|---|---|
| Pt. without arthritic Sx (n=45) | Pt. with arthritic Sx (n=44) | Healthy control (n=56) | |||
| RF (+) | 18 | 22 | 15 | 2 | <0.001 |
| (100%) | (48.9%) | (34.1%) | (3.6%) | ||
| Anti-CCP (+) | 13 | 1 | 3 | 1 | <0.001 |
| (72.2%) | (2.2%) | (6.8%) | (1.8%) | ||
| Anti-CCP (+) | |||||
| RF (+) | 13 | 0 | 2 | 1 | |
| RF (–) | 0 | 1 | 1 | 0 | |
| Anti-CCP (–) | |||||
| RF (+) | 5 | 22 | 13 | 1 | |
| RF (–) | 0 | 22 | 28 | 54 | |
Table 2.
The laboratory and radiologic characteristics of RA group patients
| N. patient | Sex | Radiologic finding | RF∗ (IU/mL) | Anti-CCP∗ (U/mL) | CRP∗ (mg/dL) | ESR∗ (mm/hr) |
|---|---|---|---|---|---|---|
| 1 | F | Soft tissue swelling | 2,640 | 417.6 | 0.8 | 37 |
| 2 | F | Bony erosion | 162 | 240.7 | 7.2 | 36 |
| 3 | F | Bony erosion | 77 | 173.1 | 1.1 | 49 |
| 4 | F | Joint effusion | 836 | 126.7 | 11.4 | 64 |
| 5 | F | Degeneration | 39 | 69.3 | 2.6 | 69 |
| 6 | F | Soft tissue swelling | 126 | 62.4 | 7.1 | 23 |
| 7 | F | Joint effusion | 94 | 45.3 | 1.0 | 33 |
| 8 | F | Bony erosion | 594 | 42.4 | 18.8 | 56 |
| 9 | F | Bony erosion | 124 | 22.3 | 7.6 | 60 |
| 10 | F | Soft tissue swelling | 273 | 15.7 | 0.6 | 28 |
| 11 | F | Bony erosion | 209 | 14.8 | 1.0 | 25 |
| 12 | F | Joint effusion | 140 | 13.5 | 1.7 | 13 |
| 13 | F | - | 89 | 13.0 | 2.6 | 83 |
| 14 | F | Bony erosion | 184 | 11.7 | 9.7 | 73 |
| 15 | F | Degeneration | 188 | 11.6 | 1.1 | 44 |
| 16 | F | Bony erosion | 170 | 11.2 | 8.1 | 72 |
| 17 | M | Bony erosion | 794 | 10.5 | 0.3 | 18 |
| 18 | M | Degeneration | 48 | 10.4 | 2.7 | 29 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download