Abstract
Background
Although malaria-specific antibody or antigen test is useful for the diagnosis of malaria infection, its cost-effectiveness has to be concerned in the area where malaria prevalence is very low. We created a panel test composed of malaria non-specific parameters, namely hematology autoanalyzer-derived results with or without addition of HDL-cholesterol data, and evaluated its usefulness in comparison with malaria-specific antibody test.
Methods
For 395 patients tested for malaria smear, the hematology parameters such as platelet count, NRBC (%) and VCS (volume, conductivity, scattering) parameters of WBC, and HDL-cholesterol data were analyzed. Statistical significance of each parameter and that of panel test with or without addition of HDL-cholesterol were evaluated.
Results
Malaria antibody test showed sensitivity of 97.1% and specificity of 99.1%. Each parameter of platelet count, NRBC (%), D parameter and HDL-cholesterol showed sensitivity of 86.8%, 41.2%, 81.8%, and 70.6%, and specificity of 85.9%, 96.3%, 72.3%, and 81.7%, respectively. Panel test without including HDL-cholesterol showed sensitivity of 91.2% and specificity of 81.6%, and that including HDL-cholesterol showed sensitivity of 91.2% and specificity of 86.2%.
Conclusions
The malaria non-specific panel test composed of hematology autoanalyzer-derived parameters showed relatively good, but slightly lower sensitivity than that of malaria-specific antibody test. It might be used as a screening test for the diagnosis of malaria infection, and addition of HDL cholesterol improved little the usefulness of the panel test.
REFERENCES
1.Milne LM., Kyi MS., Chiodini PL., Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol. 1994. 47:740–2.

2.Thomson S., Lohmann RC., Crawford L., Dubash R., Richardson H. External quality assessment in the examination of blood films for malarial parasites within Ontario, Canada. Arch Pathol Lab Med. 2000. 124:57–60.

3.Murray CK., Gasser RA Jr., Magill AJ., Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008. 21:97–110.

4.Wongsrichanalai C., Barcus MJ., Muth S., Sutamihardja A., Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg. 2007. 77(S):S119–27.

5.Park TS., Kim JH., Kang CI., Lee BH., Jeon BR., Lee SM, et al. Diagnostic usefulness of SD malaria antigen and antibody kits for differential diagnosis of vivax malaria in patients with fever of unknown origin. Korean J Lab Med. 2006. 26:241–5. (박태성, 김지훈, 강철인, 이병호, 전병렬, 이선민등. 불명열환자에서삼일열말라리아감별진단을위한 SD사말라리아항원및항체검사킷트의진단적유용성. 대한진단검사의학회지 2006;26: 241-5.).
6.Kim SW., Kim AJ., Rho JY., Shin DW., Park JS., Kim KH, et al. Use of malaria antibody test kit and clinical features in malaria patients. J Korean Soc Emerg Med. 2006. 17:210–6. (김성우, 김아진, 노준영, 신동운, 박준석, 김경환등. 말라리아의조기진단을위한말라리아항체검사의이용및임상적고찰. 대한응급의학회지 2006;17: 210-6.).
7.Choi YJ. Evaluation of n-RBC flag from Beckman Coulter STKS hematology analyzer as a screening marker for diagnosing Plasmodium vivax malaria patients. Pochun Chungmun University College of Medicine Master's Thesis. 2003. (최윤정. Plasmodim vivax 말라리아환자의조기진단을위한자동혈구분석기Beckman Coulter STKS의 n-RBC flag의 유용성 평가. 포천중문 의과대학교 보건대학원 석사학위논문 2003.).
8.de Langen AJ., van Dillen J., de Witte P., Mucheto S., Nagelkerke N., Kager P. Automated detection of malaria pigment: feasibility for malaria diagnosing in an area with seasonal malaria in northern Namibia. Trop Med Int Health. 2006. 11:809–16.

9.Shin KS., Ma KR., Lim CS. Diagnosis of malaria using automatic hematology analyzer. J Lab Med & Qual Assur. 2004. 26:171–6. (신규성, 마경란, 임채승. 자동혈액측정기를 이용한 말라리아의 진단. 임상검사와정도관리 2004;26: 171-6.).
10.Scott CS., van Zyl D., Ho E., Meyersfeld D., Ruivo L., Mendelow BV, et al. Automated detection of malaria-associated intraleukocytic haemozoin by Cell-Dyn CD4000 depolarization analysis. Clin Lab Haematol. 2003. 25:77–86.
11.Hanscheid T. Current strategies to avoid misdiagnosis of malaria. Clin Microbiol Infect. 2003. 9:497–504.
12.Briggs C., Da Costa A., Freeman L., Aucamp I., Ngubeni B., Machin SJ. Development of an automated malaria discriminant factor using VCS technology. Am J Clin Pathol. 2006. 126:691–8.

13.Fourcade C., Casbas MJ., Belaouni H., Gonzalez JJ., Garcia PJ., Pepio MA. Automated detection of malaria by means of the haematology analyser Couler GEN.S. Clin Lab Haematol. 2004. 26:367–72.
14.Kho WG. Reemergence of malaria in Korea. J Korean Med Assoc. 2007. 50:959–66. (고원규. 국내말라리아의재유행. 대한의사협회지 2007;50: 959-66.).

15.Koo KB., Cho NH., Kim SH., Won YJ., Cho HS. The clinical analysis of 79 cases of indigenous malaria in Myongji Hospital during 4 years. J Korean Acad Fam Med. 2004. 25:403–10. (구경본, 조남홍, 김선현, 원영준, 조항석. 경기 고양지역 일개병원에서 4년간 관찰된 토착형 말라리아79예의임상상의변화. 가정의학회지 2004;25: 403-10.).
16.Sin C., Lim DJ., Song TJ., Lee KC., Suh I., Yoon SY, et al. Lipid profile changes in infection of plasmodium vivax. Korean J Infect Dis. 2001. 33:58–61. (신철, 임동준, 송태진, 이규철, 서인범, 윤수영등. 삼일열말라리아의감염시혈중지질변화. 감염 2001;33: 58-61).
17.Kittl EM., Diridl G., Lenhart V., Neuwald C., Tomasits J., Pichler H, et al. HDL cholesterol as a sensitive diagnostic parameter in malaria. Wien Klin Wochenschr. 1992. 104:21–4.
18.Nilsson-Ehle I., Nilsson-Ehle P. Changes in plasma lipoproteins in acute malaria. J Intern Med. 1990. 227:151–5.

19.Tetrault GA. Incorrect test usefulness statistics in development of an automated malaria discriminant factor using VCS. Am J Clin Pathol. 2007. 128:346.
21.Wilson RF., Barletta JF., Tyburski JG. Hypocholesterolemia in sepsis and critically ill or injured patients. Crit Care. 2003. 7:413–4.
Fig. 1.
Comparison of ROC curves for panel test 1 & 2. P1 is composed of platelet count, NRBC (%) and D parameter derived from volume, conductivity, scattering indices, and P2 is composed of P1 plus HDL-cholesterol. HDL-cholesterol is not helpful to increase the sensitivity or specificity of the panel test.
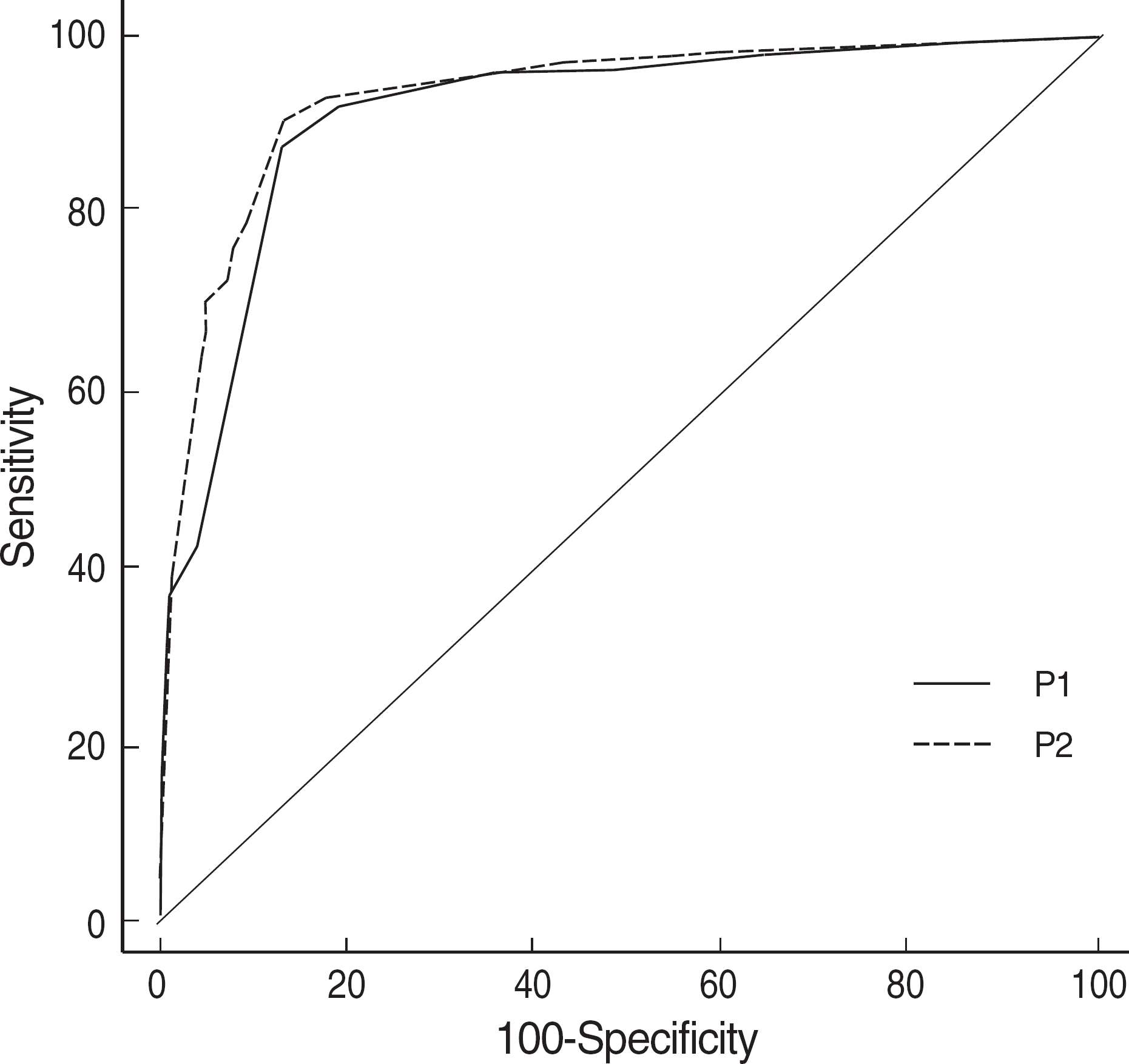
Table 1.
Statistical results for the parameters of thrombocytopenia, NRBC (%) and HDL-cholesterol
| Cut-off∗ | Sensitivity/Specificity (%) | AUC | PLR | |
|---|---|---|---|---|
| Thrombocytopenia | ≤120,000/μL | 86.8/85.9 | 0.91 | 6.3 |
| NRBC (%) | >0% | 41.2/96.3 | 0.69 | 11.2 |
| D† | >4.57 | 81.8/72.3 | 0.80 | 3.0 |
| HDL-cholesterol | ≤24 mg/dL | 70.6/81.7 | 0.80 | 3.9 |
Table 2.
Statistical significance of positional parameters of white blood cells through LH750 hematology autoanalyzer
| Neutrophil | Lymphocyte | Monocyte | |
|---|---|---|---|
| Positive/Negative∗ | Positive/Negative | Positive/Negative | |
| Vmean | 148.0±7.7/146.2±9.3 | 89.5±8.3/83.8±6.1 | 184.1±10.7/176.3±11.8 |
| NS† | P=0.0000 | P=0.0000 | |
| VSD | 23.7±2.4/23.9±3.5 | 20.6±3.4/17.4±3.1 | 25.3±3.1/23.0±3.9 |
| NS | P=0.0273 | P=0.0000 | |
| Cmean | 142.6±4.5/144.9±4.7 | 113.1±4.9/114.9±4.8 | 121.0±4.1/123.3±4.4 |
| P=0.0011 | P=0.0052 | P=0.0001 | |
| CSD | 7.2±1.2/7.1±1.4 | 14.6±3.7/12.0±3.4 | 5.8±1.4/5.4±1.2 |
| NS | P=0.0000 | P=0.0096 | |
| Smean | 141.3±10.9/144.5±8.7 | 71.8±8.3/71.0±7.7 | 89.9±6.6/90.9±5.7 |
| P=0.0105 | NS | NS | |
| SSD | 14.6±2.1/14.0±2.5 | 19.7±2.0/19.9±2.7 | 11.6±1.7/12.0±2.1 |
| NS | NS | NS |
Table 3.
Statistical results for the significantly different VCS and D parameters
| Cut-off∗ | Sensitivity/Specificity (%) | AUC | PLR | |
|---|---|---|---|---|
| Vmean-Lymphocyte | >88 | 63.2/80.4 | 0.75 | 3.2 |
| Vmean-Monocyte | >175 | 79.4/53.5 | 0.70 | 1.7 |
| VSD-Lymphocyte | >18.6 | 75.0/68.1 | 0.77 | 2.4 |
| VSD-Monocyte | >23.1 | 81.8/54.9 | 0.71 | 1.8 |
| Cmean-Neutrophil | ≤143 | 66.2/57.1 | 0.64 | 1.6 |
| Cmean-Lymphocyte | ≤112 | 50.0/66.1 | 0.60 | 1.5 |
| Cmean-Monocyte | ≤122 | 73.5/51.4 | 0.65 | 1.5 |
| CSD-Lymphocyte | >11.8 | 81.8/59.7 | 0.73 | 2.0 |
| CSD-Monocyte | >5.3 | 70.6/58.7 | 0.63 | 1.7 |
| Smean-Neutrophil | ≤141 | 52.9/69.1 | 0.59 | 1.7 |
| D† | >4.6 | 81.8/72.3 | 0.80 | 3.0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download