Abstract
Background
We evaluated the performance and false positive rate of Mediace RPR test (Sekisui, Japan), a newly introduced nontreponemal test using a chemistry autoanalyzer.
Methods
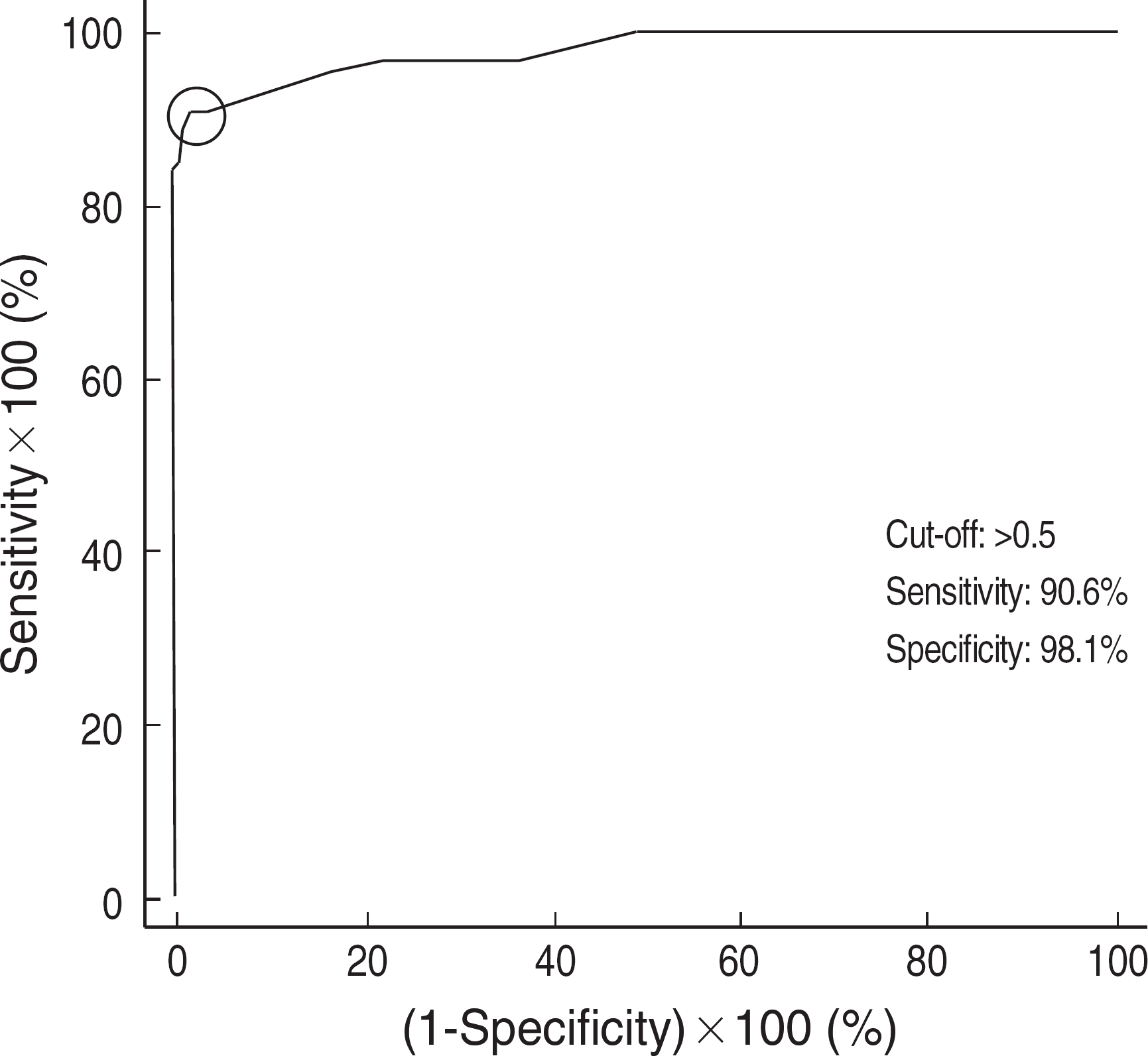
The sensitivity of Mediace RPR test was analyzed using sera from 50 patients with syphilis in different stages (8 primary, 7 secondary, and 35 latent), 14 sera positive with fluorescent treponemal antibody absorption (FTA-ABS) IgM, and 74 sera positive with conventional rapid plasma regain (RPR) card test (Asan, Korea) and also positive with Treponema pallidum hemagglutination (TPHA) test or FTA-ABS IgG test. The specificity was analyzed on 108 healthy blood donors. We also performed RPR card test on 302 sera that had been tested positive with Mediace RPR test and also performed TPHA or FTA-ABS IgG test to analyze the false positive rate of Mediace RPR test. A cutoff value of 0.5 R.U. (RPR unit) was used for Mediace RPR test.
Results
Mediace RPR test on syphilitic sera of different stages (primary, secondary, and latent stages) and FTA-ABS IgM positive sera showed a sensitivity of 100%, 100%, 82.9% and 100%, respectively. Among the 74 sera positive with conventional RPR card test and TPHA or FTA-ABS IgG test, 55 were positive with Mediace test. The specificity of Mediace RPR test on blood donors was 97.2%. Among the 302 sera positive with Mediace RPR test, 137 sera (45.4%) were negative by RPR card and TPHA/FTA-ABS IgG tests.
Conclusions
Although the sensitivities of Mediace RPR were good for primary and secondary syphilis, due to its high negative rate of Mediace RPR over the conventional RPR positive samples, further studies are necessary whether it can replace conventional nontreponemal test for screening purpose. Moreover, in view of the high false positive rate, positive results by Mediace RPR test should be confirmed with treponemal tests.
Go to : 
REFERENCES
1.Smith MB., Hayden RT., Persing DH., Woods GL. Spirochete infections. Henry JB, editor. Clinical Diagnosis and Management by Laboratory Methods. 20th ed.Philadelphia: W.B. Saunders;2001. p. 1131–43.
2.Kinjyo T., Nago T., Kiyama KS., Ohshiro M., Nagamine T., Yamane N. Laboratory-based evaluation of latex-agglutination turbidemetric assay by Mediace RPR on P Module of Hitachi autoanalyzer 7600 to quantitatively determine serum RPR antibody. Jap J Clin Lab Assoc. 2005. 30:257–62.
3.Kawai K., Osato K. The possibility of assessing the stage of infection by using Sekisui's automated TPLA and RPR. J Clin Lab Instrum Reag. 2003. 26:301–4.
4.Kume T. The clinical utility of quantitation for anti-lipoidal antibody in serum using a latex agglutination immunoassay on an automatic analyzer. Jap J Clin Lab Assoc. 2003. 28:254–5.
5.Osato K., Nagao T., Inuzumi K., Araki H., Kawai K. Clinical evaluation of latex agglutination test kits for detecting anti-syphilitic lipoidal antibodies and anti-treponemal antibodies. Jap J Sex Transm Dis. 2002. 13:124–30.
6.Huh HJ., Lee KK., Kim ES., Chae SL. Analysis of Positive Results in Mediace Rapid Plasma Reagin and Treponema pallidum Latex Agglutination as the Automated Syphilis Test. Korean J Lab Med. 2007. 27:324–9. (허희진, 이교관, 김의석, 채석래. 자동화 매독 검사인 Medicae Rapid Pasma Reagin과 Treponema pallidum Latex Agglutination 양성결과분석. 대한진단검사의학회지 2007;27: 324-9.).

7.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997. 46(RR-10):1–55.
8.Wicher K., Horowitz HW., Wicher V. Laboratory methods of diagnosis of syphilis for the beginning of the third millennium. Microbes Infect. 1999. 1:1035–49.

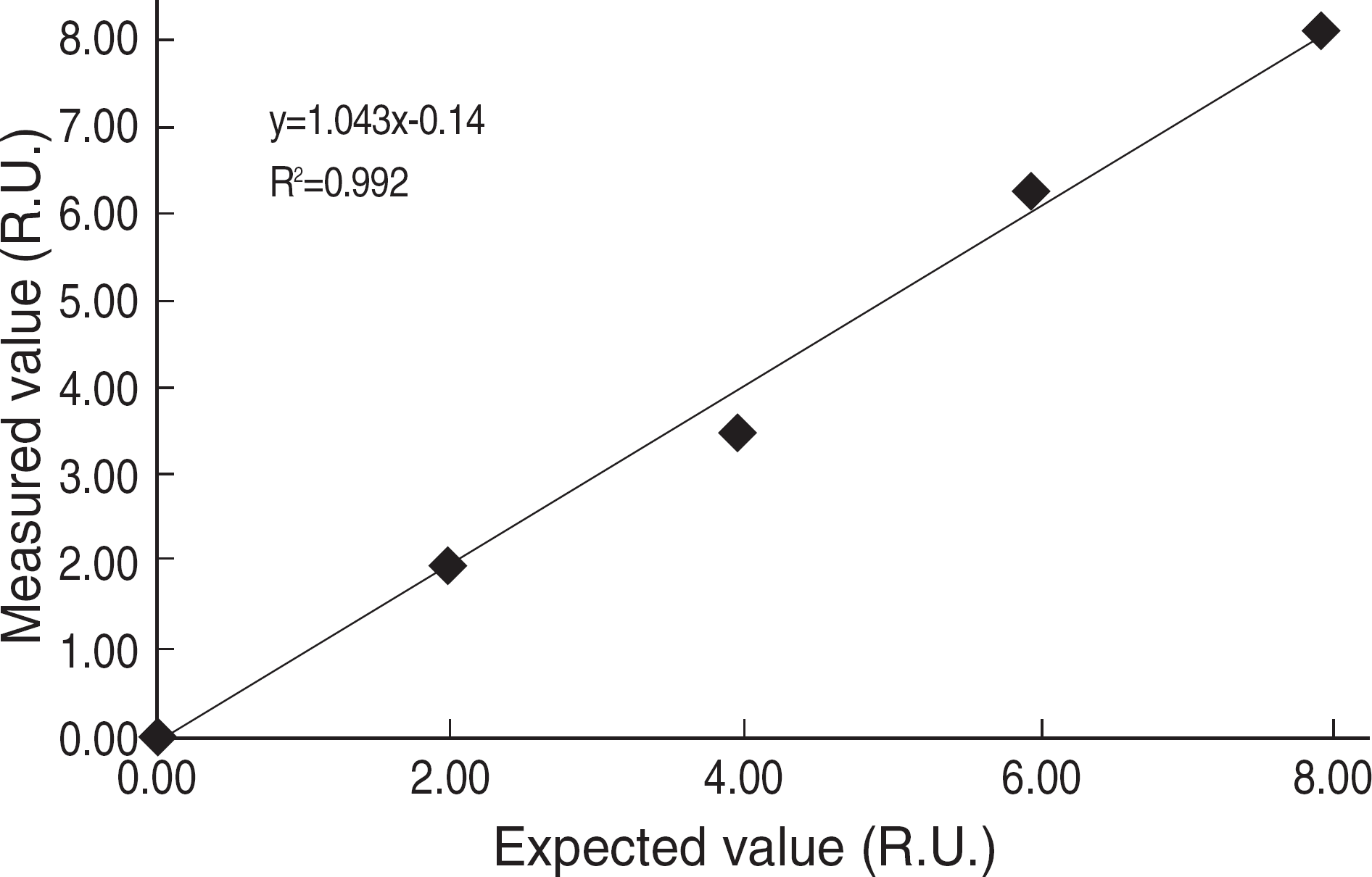
9.National Committee for Clinical Laboratory Standards. Preliminary evaluation of quantitative clinical laboratory methods; Approved guideline EP10-A. Wayne, PA: NCCLS;1998.
10.National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative measurement procedures; a statistical approach. Approved guideline EP6-A. Wayne, PA: NCCLS;2003.
11.Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006. 55(RR-11):1–94.
12.Screening Guidelines Steering Group. Sexually Transmitted Infections: UK National Screening and Testing Guidelines, Aug 2006. London: Screening Guidelines Steering Committee;2006.
13.The Syphilis Guideline Revision Group. European Guideline on the Management of Syphilis 2007. International Union Against Sexually Transmitted Infections. 2007. http://www.iusti.org/regions/europe/euroguidelines.htm. , updated in Feb 2008).
Go to : 
Table 1.
Sensitivity and specificity of Mediace RPR test with different cutoff values (1.0 R.U. vs 0.5 R.U.)
Table 2.
Analysis of false positive results of Mediace RPR test
Table 3.
Distribution of R.U. values of Mediace RPR-positive samples with possible interpretation (N=243)
Table 4.
Distribution of titers of RPR card test on Mediace RPR test negative and RPR card/TPHA or FTA-ABS IgG positive samples
| Titer of RPR card | Cutoff 0.5 (N=19) | Cutoff 1.0 (N=32) |
|---|---|---|
| WR | 6 | 8 |
| 1:1 | 4 | 6 |
| 1:2 | 6 | 13 |
| 1:4 | 3 | 5 |
Table 5.
Precision of Mediace RPR test
| Low (1.0 R.U.) | Medium (4.5 R.U.) | High (8.0 R.U.) | |
|---|---|---|---|
| Within-run CV (%) | 8.3 | 3.3 | 1.6 |
| Total CV (%) | 12.8 | 3.5 | 1.7 |
Table 6.
Changes of Mediace RPR test (R.U.) and the titer of RPR card after treatment
| Stage of syphilis | No. case monitored | Pre-tx. | Post-tx. (3-6 months) | Post-tx. (9-12 months) | |||
|---|---|---|---|---|---|---|---|
| Mediace RPR∗ | RPR card | Mediace RPR∗ | RPR card | Mediace RPR∗ | RPR card | ||
| Primary | 1 | 3.9 | 1:32 | 0.4 | NT | ||
| 2 | 3.9 | 1:32 | <0.1 | NT | |||
| 3 | 2.4 | NT | 0.8 | 1:2 | |||
| Secondary | 1 | 2.1 | >1:32 | 5.5 | 1:8 | ||
| 2 | 2.6 | >1:32 | 2.8 | 1:2 | |||
| 3 | 2.8 | 1:32 | 0.9 | 1:1 | |||
| 4 | 3.6 | 1:32 | <0.1 | NT | |||
| 5 | 4.6 | 1:8 | NT | NT | 0.2 | NT | |
| Latent | |||||||
| treated | 1 | 3.8 | 1:32 | 4.2 | 1:8 | 2.6 | 1:8 |
| untreated | 1 | 4.0 | 1:32 | 1.8 | 1:1 | ||
| 2 | 4.9 | 1:16 | 0.4 | NT | |||
| 3 | 5.2 | 1:8 | 4.5 | 1:4 | |||
| 4 | 4.4 | NT | 4.2 | 1:4 | 4.7 | 1:2 | |
| 5 | 2.3 | 1:32 | 7.0 | 1:16 | 4.0 | 1:2 | |
| 6 | 0.6 | NT | 0.7 | WR | |||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download