Abstract
Background
Infections caused by mycobacteria have been significantly increasing. Due to the difficulty of making a decision about the pathogenicity of mycobacteria, species-level identification is very important for patients’ diagnosis and treatment. The purpose of this study was to identify mycobacteria species using a high performance liquid chromatography (HPLC) method and to provide an initial database for the distribution of mycobacteria in Korea.
Methods
Acid fast bacteria isolated from 3,107 clinical specimens were identified by mycolic acid analysis using HPLC. The HPLC patterns were compared with those of standard mycobacteria species.
Results
The HPLC patterns were divided into single, double, and triple cluster groups, each group comprising 9, 20, and 4 species, respectively. Mycobacteria and non-tuberculous mycobacteria (NTM) were identifies by HPLC at the rates of 99.5% and 95.6%, respectively. NTM was isolated in 12.4% of the mycobacteria positive specimens. This study also found that there were 20 different NTM species with the distribution of each species ranging from 0.3% to 15.9% of the total NTM. While the rate of NTM has been increasing in Korea, M. avium-intracellulare, M. fortuitum, and M. chelonae are relatively decreasing, and M. kansasii and M. gordonae are relatively increasing.
REFERENCES
1.Raviglione MC., Snider DE Jr., Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995. 273:220–6.
2.World Health Organization. Strategic framework to decrease the burden of TB/HIV. WHO/CDS/TB/2002.296, WHO/HIV_AIDS/02.2. 2002.
3.World Health Organization. Global Tuberculosis Control. WHO Report. Geneva, Switzerland. WHO/CDS/TB/2002.295. 2002.
4.Korean academy of tuberculosis and respiratory disease. National survey of mycobacterial disease other than tuberculosis in Korea. Tuberc Respir Dis. 1995. 42:277–94. (대한결핵 및 호흡기학회. 비결핵항산균증 전국 실태조사. 결핵 및 호흡기질환 1995;42: 277-94.).
5.Falkinham JO 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996. 9:177–215.

6.Horsburgh CR Jr. Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991. 324:1332–8.
7.Hoffner SE. Pulmonary infections caused by less frequently encountered slow-growing environmental mycobacteria. Eur J Clin Microbiol Infect Dis. 1994. 13:937–41.

8.Prince DS., Peterson DD., Steiner RM., Gottlieb JE., Scott R., Israel HL, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989. 321:863–8.
9.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979. 119:107–59.
10.Mori T. Atypical mycobacteriosis. Nippon Rinsho. 2001. 59(S):S197–204.
11.Holdiness MR. Atypical mycobacterial infections. J La State Med Soc. 1990. 142:31–8.
12.Kruger M. Atypical mycobacterium infections. Derm Beruf Umwelt. 1990. 38:109–17.
13.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997. 156:S1–25.
14.Phillips MS., von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis. 2001. 33:1363–74.

15.Park CM., Heo SR., Park KU., Song JH., Lee JH., Lee CT, et al. Isolation of Nontuberculous mycobacteria using polymerase chain reaction-restriction fragment length polymorphism. Korean J Lab Med. 2006. 26:161–7. (박철민, 허세란, 박경운, 송정한, 이재호, 이춘택 등. 중합효소연쇄반응-제한절편길이다형성을 이용한 비결핵항산균의 분리. 대한진단검사의학회지 2006;26: 161-7.).

16.Nah J., Huh JW., Lee SH., Kim BC., Koh YS., Pai CH. Identification of mycobacterium tuberculosis complex using a gene probe method. Korean J Clin Pathol. 1997. 17:71–8. (나준, 허정원, 이성희, 김봉철, 고윤석, 배직현. Gene Probe 법에 의한 Mycobacterium tuberculosis com- plex의 동정. 대한임상병리학회지 1997;17: 71-8.).
17.National Committee for Clinical Laboratory Standards. Molecular diagnostic methods for infectious diseases. Proposed guideline. 2nd ed.MM3-P2. Wayne, Pa: NCCLS;2005.
18.Noordhoek GT., van Embden JD., Kolk AH. Questionable reliability of the polymerase chain reaction in the detection of Mycobacterium tuberculosis. N Engl J Med. 1993. 329:2036.

19.Leao SC., Sampaio JL., Martin A., Palomino JC., Portaels F. Profiling Mycobacterium ulcerans with hsp65. Emerg Infect Dis. 2005. 11:1795–6.
20.Reisner BS., Gatson AM., Woods GL. Use of Gen-Probe AccuProbes to identify Mycobacterium avium complex, Mycobacterium tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J Clin Microbiol. 1994. 32:2995–8.
21.Burman WJ., Reves RR. Review of false positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin Infect Dis. 2000. 31:1390–5.
22.Butler WR., Guthertz LS. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin Microbiol Rev. 2001. 14:704–26.
23.Butler WR., Jost KC Jr., Kilburn JO. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991. 29:2468–72.

24.Butler WR,Floyd MM, et al. Mycolic acid pattern standards for HPLC identification of mycobacteria. Atlanta, Ga: Centers for Disease Control and Prevention, US department of health and human services. 1999. 1–86.
25.Butler WR, Floyd MM, editors. Standardized method for HPLC identification of mycobacteria. Atlanta, Ga: US department of health and human services;1996. p. 1–99.
26.Jeong J., Lee SH., Jeong US., Chang CH., Kim SR. Identification of mycobacteria using high performance liquid chromatography in clinical specimens. Korean J Clin Microbiol. 2004. 7:148–55. (정윤성, 이선호, 정의석, 장철훈, 김성률. 임상검체에서 High performance liquid chromatography법을 이용한 mycobacteria의 동정. 대한임상미생물학회지 2004;7: 148-55.).
27.Euzeby JP, editor. ed.List of bacterial names with standing in nomenclature, 1998. Society for Systematic and Veterinary Bacteriology;London: 2002.
28.Tortoli E., Bartoloni A., Bottger EC., Emler S., Garzelli C., Magliano E, et al. Burden of unidentifiable mycobacteria in a reference laboratory. J Clin Microbiol. 2001. 39:4058–65.

29.Field SK., Cowie RL. Lung disease due to the more common nontuberculous mycobacteria. Chest. 2006. 129:1653–72.

30.Olivier KN. Nontuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med. 1998. 4:148–53.
31.Dobos KM., Quinn FD., Ashford DA., Horsburgh CR., King CH. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999. 5:367–78.

32.Inderlied CB., Kemper CA., Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993. 6:266–310.
33.Primm TP., Lucero CA., Falkinham JO 3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004. 17:98–106.

34.O'Brien RJ., Geiter LJ., Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987. 135:1007–14.
35.Kim KS., Shin YD., Ahn JW., Lee SK. Unclassified mycobacteria in Korea epidermiological study on unclassified infection using purified tuberculin protein. Tuberc Respir Dis. 1966. 13:5–20. (김경수, 신용달, 안재원, 이성관. Unclassified mycobacteria의 역학적 연구. 결핵 및 호흡기질환 1966;13: 5-20.).
36.Lee SK., Shin YD. Epidemiologic study on the unclassified mycobacteria in Korea. Tuberc Respir Dis. 1967. 14:12–38. (이성관 및 신용달. 비정형항산균 감염의 역학적 연구. 결핵 및 호흡기질환 1967;14: 12-38.).
37.Kim SC., Kim SC. A study on unclassified mycobacteria isolated from human sputa. Tuberc Respir Dis. 1970. 17:33–42. (김성진 및 김상재. 객담에서 분리된 미분류항산균에 관한 연구. 결핵 및 호흡기질환 1970;17: 33-42.).

38.Centers for Disease Control and Prevention. Nontuberculous mycobacteria reported to the public health laboratory information system by state public health laboratories United States, 1993-1996. Atlanta: Centers for Disease Control and Prevention;1999.
39.Koh WJ., Kwon OJ., Jeon K., Kim TS., Lee KS., Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–8.

40.Yim JJ., Park YK., Lew WJ., Bai GH., Han SK., Shim YS. Mycobacterium kansasii pulmonary diseases in Korea. J Korean Med Sci. 2005. 20:957–60.
Fig. 1.
Characteristic HPLC chromatograms of standard Mycobacterium species with single-cluster peak patterns. (A) M. asiaticum. (B) M. bovis. (C) M. gastri. (D) M. gordonae. (E) M. kansasii. (F) M. marinum. (G) M. szulgai. (H) M. trivial and (I) M. tuberculosis.
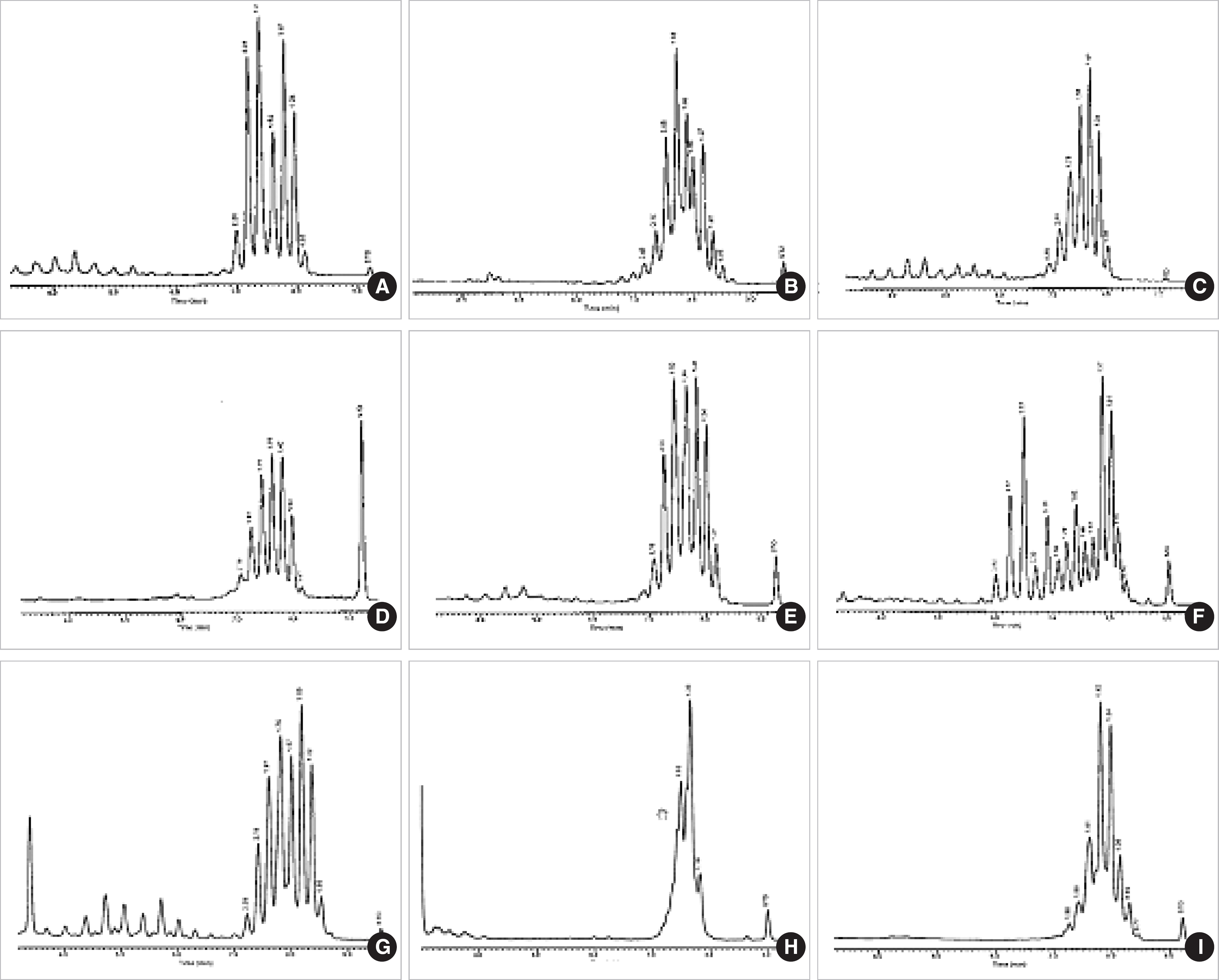
Fig. 2.
Characteristic HPLC chromatograms of standard Mycobacterium species with double-cluster peak patterns. (A) M. abscessus. (B) M. acapulsensis. (C) M. agri. (D) M. avium. (E) M. celatum. (F) M. chelonae. (G) M. diernhoferi. (H) M. flavescens. (I) M. fortuitum. (J) M. gilvum. (K) M. intracellulare. (L) M. mucogenicum. (M) M. nonchromogenicum. (N) M. peregrinum. (O) M. phlei. (P) M. porcinum. (Q) M. scrofulaceum. (R) M. smegmatis. (S) M. terrae and (T) M. xenopi.
Fig. 3.
Characteristic HPLC chromatograms of standard Mycobacterium species with triple-cluster peak patterns. (A) M. austroafricanum. (B) M. pulveris. (C) M. simiae and (D) M. vaccae.
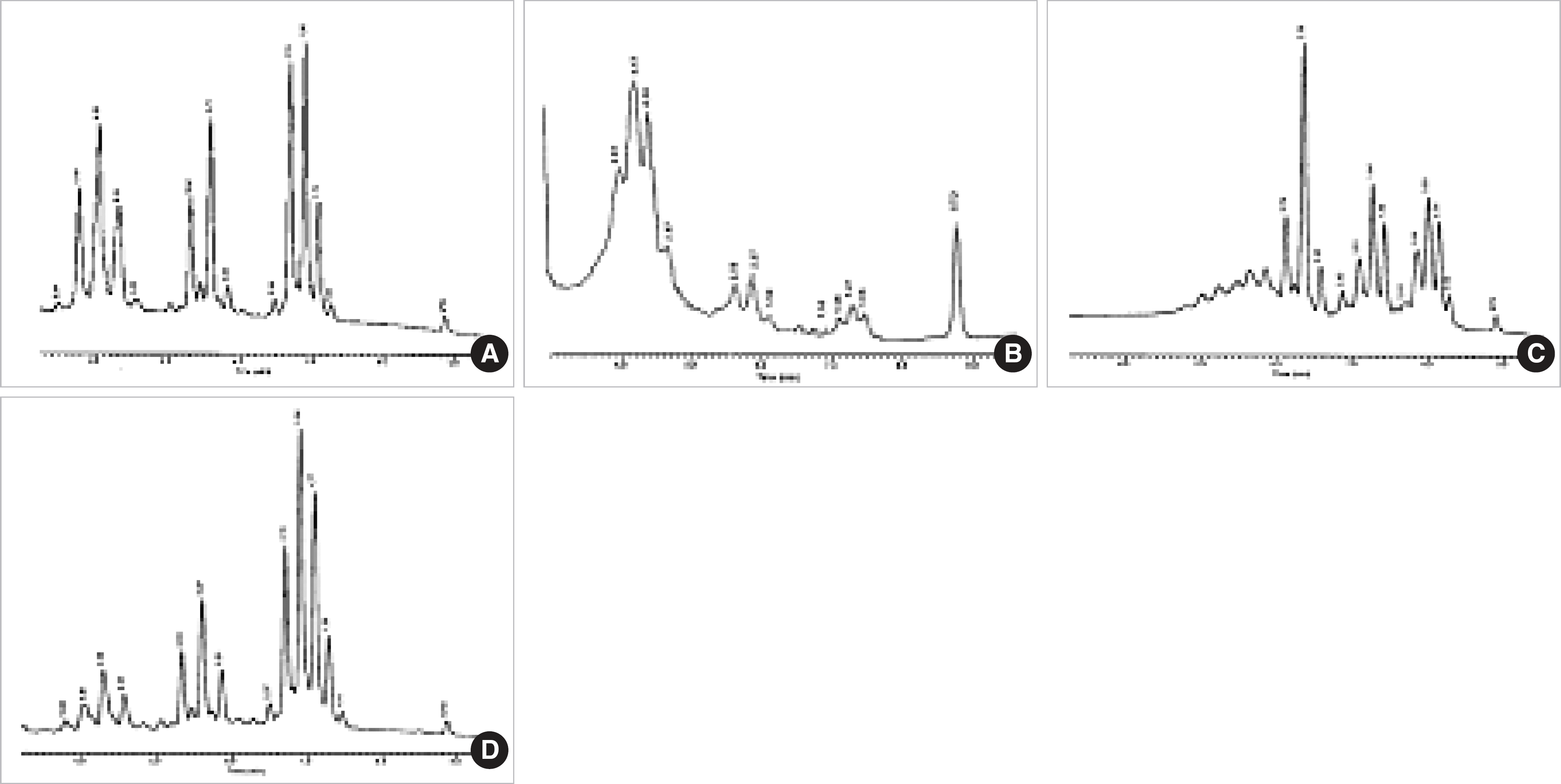
Table 1.
Standard mycobacterial isolates used in this study
Table 2.
Isolation rates of M. tuberculosis and nontuberculous mycobacteria in AFB culture positive specimens using HPLC method
| M. tuberculosis | Nontuberculous mycobacteria | ||
|---|---|---|---|
| Patients | Specimens | Patients | Specimens |
| N (%) | N (%) | N (%) | N (%) |
| 1,141 (85.0) | 2,723 (87.6) | 201 (15.0) | 384 (12.4) |
Table 3.
Distribution of nontuberculous Mycobacterium species in clinical specimens
Table 4.
Annual incidence of species comprising more than 5% of total nontuberculous mycobacteria




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download