Abstract
Background
This study aimed to analyze the influence of the interruption of agitation and removal of leukocytes on platelet concentrates (PCs), and determine the maximum amount of time the agitation could be interrupted without impairing PCs’ effectiveness during the storage period.
Methods
Four ABO-identical random donor platelets agitated for 24 hr were pooled, and divided into 4 units, and 2 units of them were leukoreduced. Then 52 pooled units were categorized into 4 groups, non-leukoreduced continuous agitation (Non-LRCA), non-leukoreduced interrupted agitation (Non-LRIA), leukoreduced continuous agitation (LRCA), and leukoreduced interrupted agitation (LRIA), and preserved for 6 days (total 7 days). Mean platelet volume (MPV), pH, HCO3−, pO2, pCO2, CD62P, CD61, glucose, lactate, ammonia and free fatty acid were measured during the period.
Results
Starting from the Day 4, the pH and HCO3− of Non-LRIA group begun to decrease while the amount of lactate production, glucose consumption, and MPV increased compared to the Non-LRCA group (P<0.01). An increase in pO2 level was observed in the interrupted agitation groups as the storage period prolonged (P<0.01). The pH levels of all the units in the agitation groups remained higher than 6.4 up to Day 7, while those of the non-leukoreduction group did so only up to Day 2, but those of leukoreduction in the interrupted agitation groups did so up to Day 4.
Conclusions
The interruption of agitation reduced the platelet's capacity to utilize oxygen, increasing lactate amount and reducing pH level. However, the in vitro parameters of the Non-LRIA and Non-LRCA groups on Day 2 were similar to each other and the pH level remained at 6.4 or higher, making one day of agitation interruption possible after 24 hr of agitation. With leukocytes removed, the effective agitation interruption period may become longer.
Go to : 
REFERENCES
1.Simon TL., Nelson EJ., Carmen R., Murphy S. Extension of platelet concentrate storage. Transfusion. 1983. 23:207–12.

2.Murphy S., Gardner FH. Platelet storage at 22 degrees C: role of gas transport across plastic containers in maintenance of viability. Blood. 1975. 46:209–18.

3.Murphy S., Sayar SN., Gardner FH. Storage of platelet concentrates at 22 degrees C. Blood. 1970. 35:549–57.
4.Hunter S., Nixon J., Murphy S. The effect of interruption of agitation on platelet quality during storage for transfusion. Transfusion. 2001. 41:809–14.
5.van der Meer PF., Gulliksson H., Aubuchon JP., Prowse C., Richter E., de Wildt-Eggen J. Interruption of agitation of platelet concentrates: effects on in vitro parameters. Vox Sang. 2005. 88:227–34.
6.Kilkson H., Holme S., Murphy S. Platelet metabolism during storage of platelet concentrates at 22 degrees C. Blood. 1984. 64:406–14.

7.Hagberg IA., Akkok CA., Lyberg T., Kjeldsen-Kragh J. Apheresis-induced platelet activation: comparison of three types of cell separators. Transfusion. 2000. 40:182–92.
8.Brecher ME, editor. Technical manual. 15th ed.Bethesda: American Association of Blood Banks;2005. p. 189–99.
9.Guide to the preparation use and quality assurance of blood components. Recommendation No. R(95)15. 12th ed.Strasbourg: Council of Europe;2006. p. 121–31.
10.de Wildt-Eggen J., Schrijver JG., Bouter-Valk HJ., Fijnheer R., Bins M., van Prooijen HC. Improvement of platelet storage conditions by using new polyolefin containers. Transfusion. 1997. 37:476–81.

11.Moroff G., George VM. The maintenance of platelet properties upon limited discontinuation of agitation during storage. Transfusion. 1990. 30:427–30.

12.Wallvik J., Stenke L., Akerblom O. The effect of different agitation modes on platelet metabolism, thromboxane formation, and alpha-granular release during platelet storage. Transfusion. 1990. 30:639–43.

13.Rinder HM., Murphy M., Mitchell JG., Stocks J., Ault KA., Hillman RS. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991. 31:409–14.

14.Fijhneer R., Modderman PW., Veldman H., Ouwehand WH., Nieuwenhuis HK., Roos D, et al. Detection of platelet activation with monoclonal antibodies and flow cytometry. Changes during platelet storage. Transfusion. 1990. 30:20–5.

15.Nieuwenhuis HK., van Oosterhout JJ., Rozemuller E., van Iwaarden F., Sixma JJ. Studies with a monoclonal antibody against activated platelets: evidence that a secreted 53,000-molecular weight lyso-some-like granule protein is exposed on the surface of activated platelets in the circulation. Blood. 1987. 70:838–45.

16.Kutlay S., Ilhan O., Arslan O., Beksac M. Influence of storage time on activation of platelets collected with CS 3000 Plus and Cobe Spectra using platelet storage containers. Ther Apher. 2002. 6:82–5.

17.Cesar J., DiMinno G., Alam I., Silver M., Murphy S. Plasma free fatty acid metabolism during storage of platelet concentrates for transfusion. Transfusion. 1987. 27:434–7.

18.Holme S., Heaton A., Momoda G. Evaluation of a new, more oxygen-permeable, polyvinylchloride container. Transfusion. 1989. 29:159–64.

19.Snyder EL., Ezekowitz M., Aster R., Murphy S., Ferri P., Smith E, et al. Extended storage of platelets in a new plastic container. II. In vivo response to infusion of platelets stored for 5 day. Transfusion. 1985. 25:209–14.
21.Holme S., Murphy S. Platelet storage at 22 degrees C for transfusion: interrelationship of platelet density and size, medium pH, and viability after in vivo infusion. J Lab Clin Med. 1983. 101:161–74.
22.Fijnheer R., Pietersz RN., de Korte D., Roos D. Monitoring of platelet morphology during storage of platelet concentrates. Transfusion. 1989. 29:36–40.

Go to : 
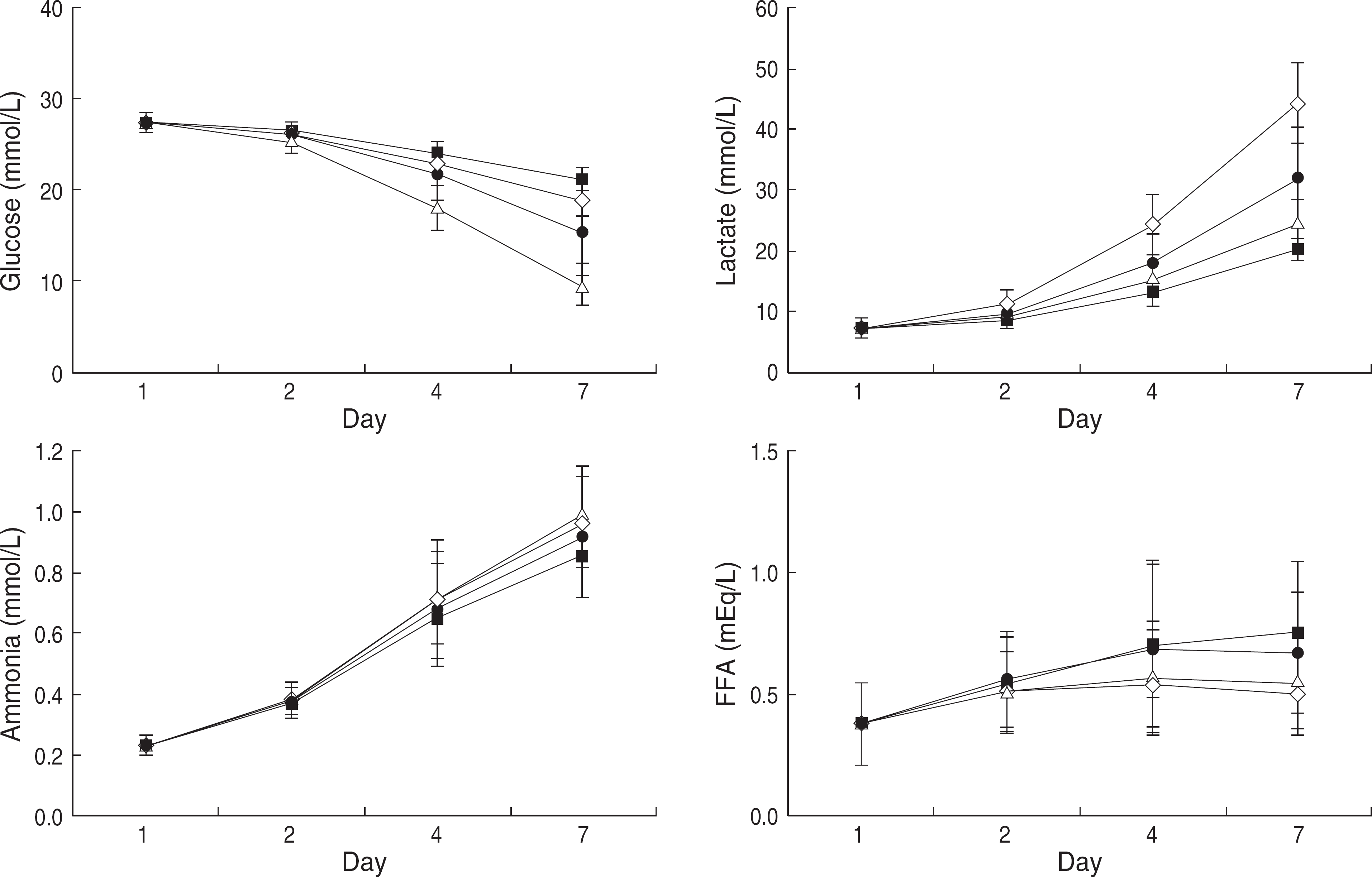 | Fig. 1.Metabolic measurements of platelets concentrations with a Non-LRCA (□), Non-LRIA (Δ), LRCA (▪) and LRIA (□) during the storage period. The results show mean standard deviation (SD) and error bars (n=13). (Glucose and Lactate: Day 4, 7 P<0.01) P values were calculated by Mann-Whitney test and compared between groups in the same storage period. Abbreviations: Non-LRCA, Non-LRIA, LRCA, LRIA, See Table 1. |
Table 1.
Changes of in vitro parameters among groups during storage of platelet concentrates
| MPV (fL) (n=13) | HCO3 (mmol/L) (n=13) | pO2 (mmHg) (n=13) | pCO2 (mmHg) (n=13) | pH (n=13) | CD62P (%) (n=13) | |
|---|---|---|---|---|---|---|
| Day 1 | 6.6±0.31 | 17.3±1.24 | 67.6±44.55 | 61.7±8.24 | 7.25±0.09 | 0.47±0.26 |
| Day 2 | ||||||
| Non-LRCA | 6.9±0.28 | 15.0±1.53 | 21.2±13.17 | 64.2±8.16 | 7.2±0.09 | 0.2±0.11 |
| Non-LRIA | 6.9±0.27 | 13.8±1.74 | 28.0±22.93∗ | 71.6 ± 11.64‡ | 7.1±0.12 | 0.3±0.17 |
| P=0.390 | P=0.057 | P=0.614 | P=0.057 | P=0.050 | P=0.977 | |
| LRCA | 6.8±0.22 | 15.1±1.09 | 55.8±42.33 | 51.5±7.37 | 7.3±0.008 | 0.3±0.24 |
| LRIA | 6.8±0.28 | 14.2±1.41 | 54.6 ±41.32† | 55.1 ±10.50‡ | 7.2±0.12 | 0.3±0.15 |
| P=0.390 | P=0.223 | P=0.920 | P=0.418 | P=0.243 | P=0.932 | |
| Day 4 | ||||||
| Non-LRCA | 7.0±0.19 | 10.5±2.47 | 25.4±18.90 | 51.8±4.04 | 7.1±0.13 | 0.3±0.20 |
| Non-LRIA | 7.6±0.44‡ | 5.1 ±2.56§ | 47.5±24.10∗ | 61.5 ±8.00† | 6.65 ±0.25‡ | 0.5±0.32 |
| P=0.000 | P=0.000 | P=0.017 | P=0.004 | P=0.000 | P=0.319 | |
| LRCA | 7.0±0.25 | 11.3±1.68 | 59.8±39.60 | 42.5±7.10 | 7.22±0.10 | 0.4±0.19 |
| LRIA | 7.1 ±0.33‡ | 8.1 ±3.08§ | 65.9 ±33.26‡ | 50.0 ±9.60† | 7.00 ±0.26† | 0.4±0.25 |
| P=0.479 | P=0.003 | P=0.579 | P=0.016 | P=0.004 | P=0.630 | |
| Day 7 | ||||||
| Non-LRCA | 7.1±0.23 | 5.7±1.72 | 24.3±18.05 | 44.0±3.95 | 6.89±0.17 | 1.0±0.87 |
| Non-LRIA | 7.9±0.33 | 0.3±0.54§ | 149.5 ±34.18∗,§ | 14.4±8.91§ | 5.95 ±0.30‡ | 7.8±4.94 |
| P=0.000 | P=0.000 | P=0.000 | P=0.000 | P=0.000 | P=0.000 | |
| LRCA | 7.1±0.20 | 6.8±1.15 | 60.2±40.56 | 36.7±7.39 | 7.07±0.14 | 1.4±1.23 |
| LRIA | 7.6±0.37 | 2.5±2.36§ | 107.2±45.65†,§ | 24.8±10.51§ | 6.51 ±0.51† | 4.0±5.75 |
| P=0.000 | P=0.000 | P=0.007 | P=0.001 | P=0.001 | P=0.525 |
Day 1, right after pooling; Day 2, one day after pooling; Day 4, three day after pooling; Day 7, 6 day after pooling.
P values were calculated by Mann-Whitney test and compared between groups in the same storage period.
Table 2.
Correlation analysis of in vitro parameters according to pH change




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download