Abstract
Background
Methods
Results
Conclusions
REFERENCES
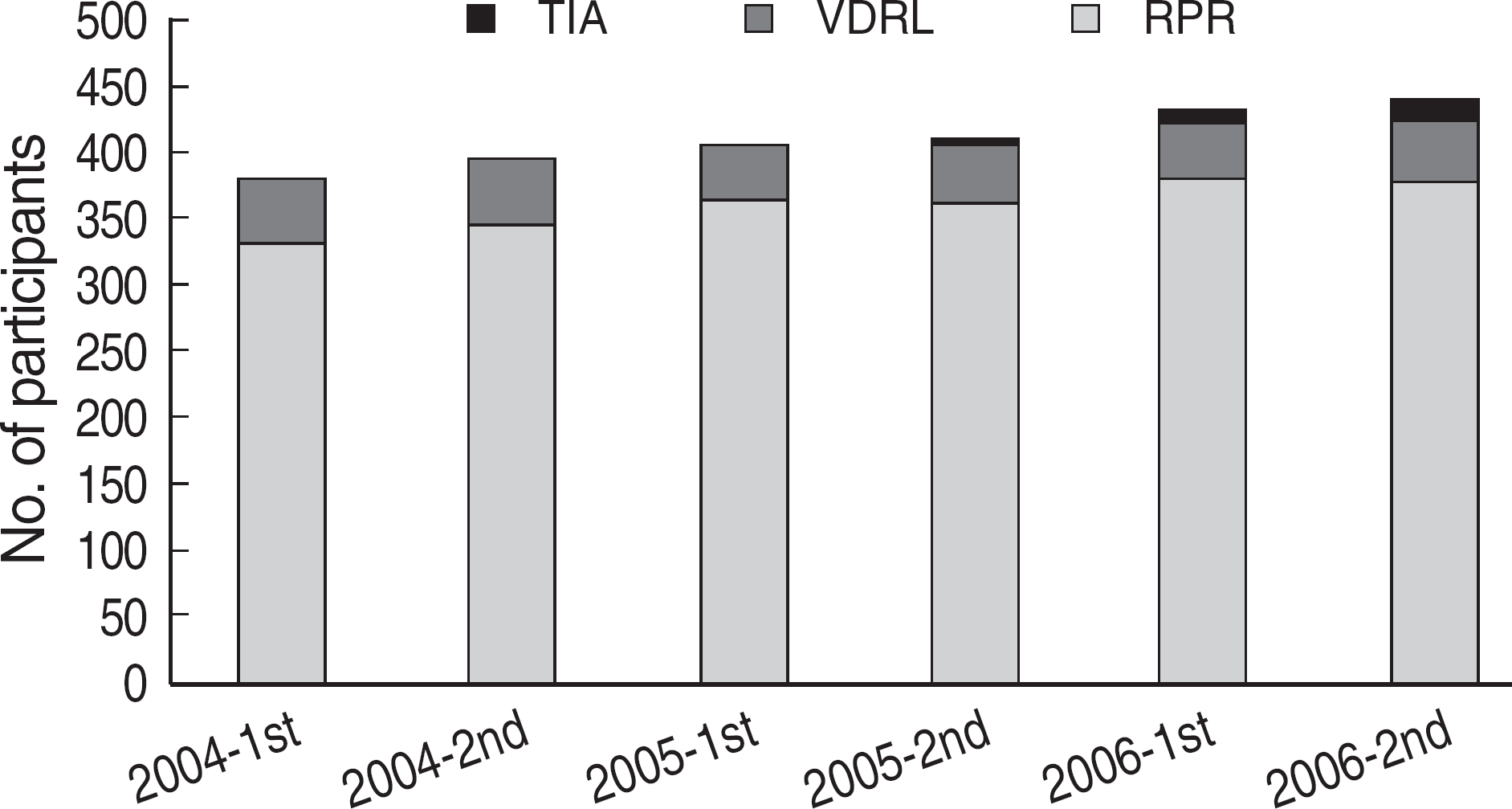 | Fig. 1.Distribution of the methods of non-treponemal tests used in Korea (2004-2006). Numbers of the participants who used each method were shown in each survey. Abbreviations: No., number; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory; TIA, turbidoimmunoassay. |
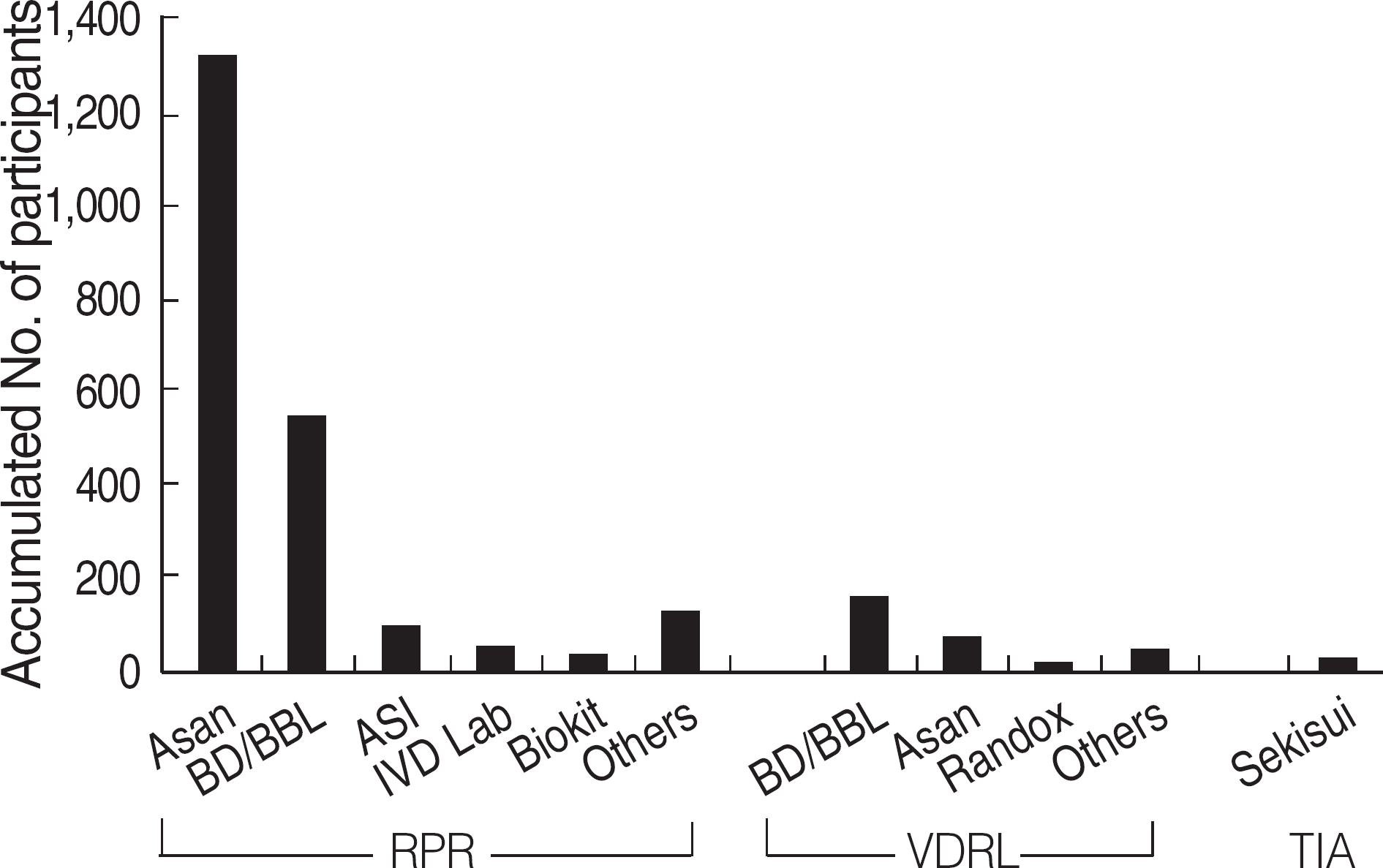
 | Fig. 2.Distribution of the manufacturers of non-treponemal tests used in Korea (2004-2006). Accumulated numbers of the participants who used each method and kit during the 3 yrs of surveys (2004-2006) were shown. Abbreviations: No., number; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory; TIA, turbidoimmunoassay; BD/BBL, Becton Dickinson. |
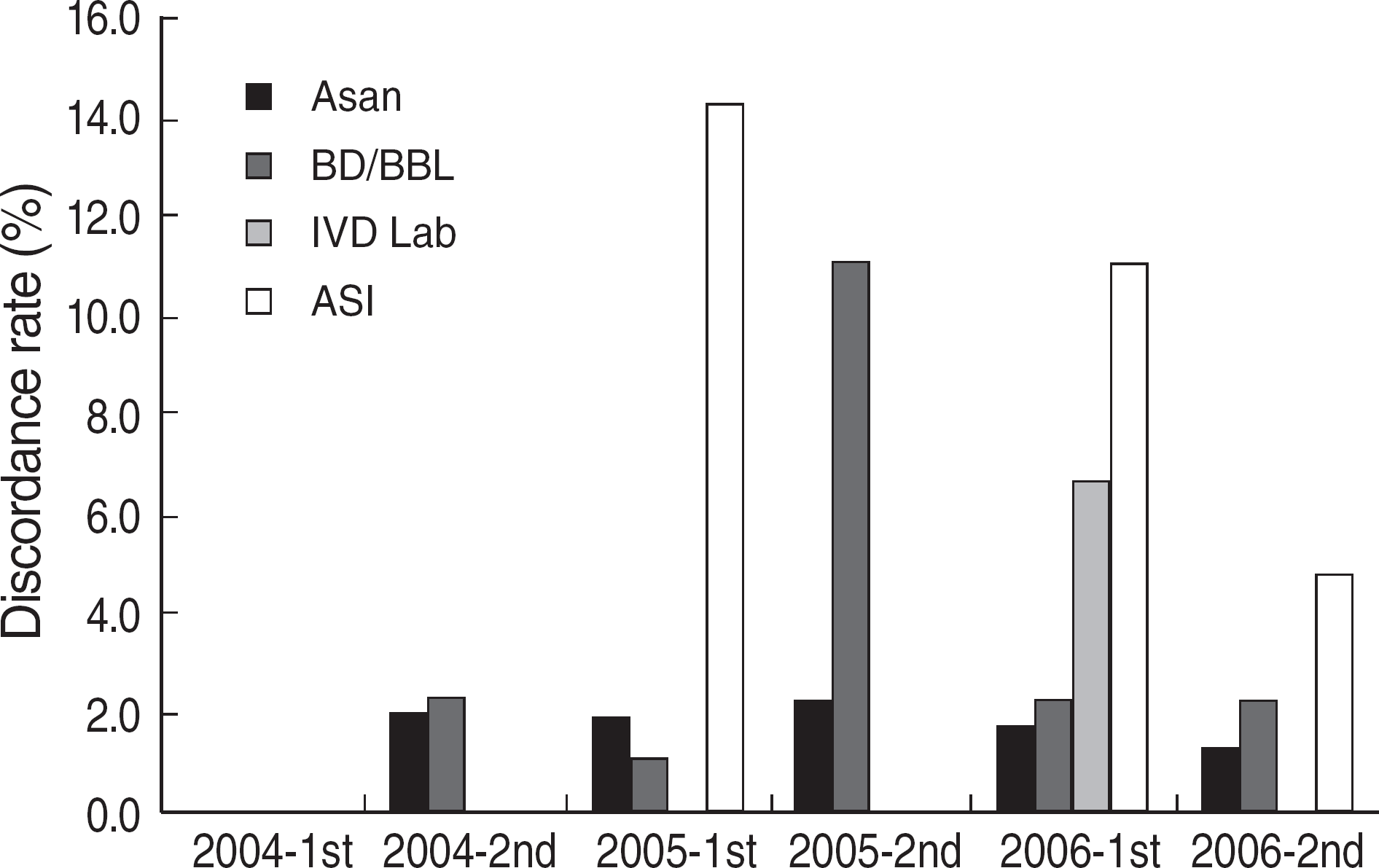
 | Fig. 3.Discordance rates of RPR test in Korea (2004-2006). Discordance rate was calculated as a number of participants with discordant results divided by a number of total participants. A discordant result was defined as a result different from the consensus (more than 80% of participants). Abbreviations: BD/BBL, Becton Dickinson; ASI, Arlington Scientific, Inc. |
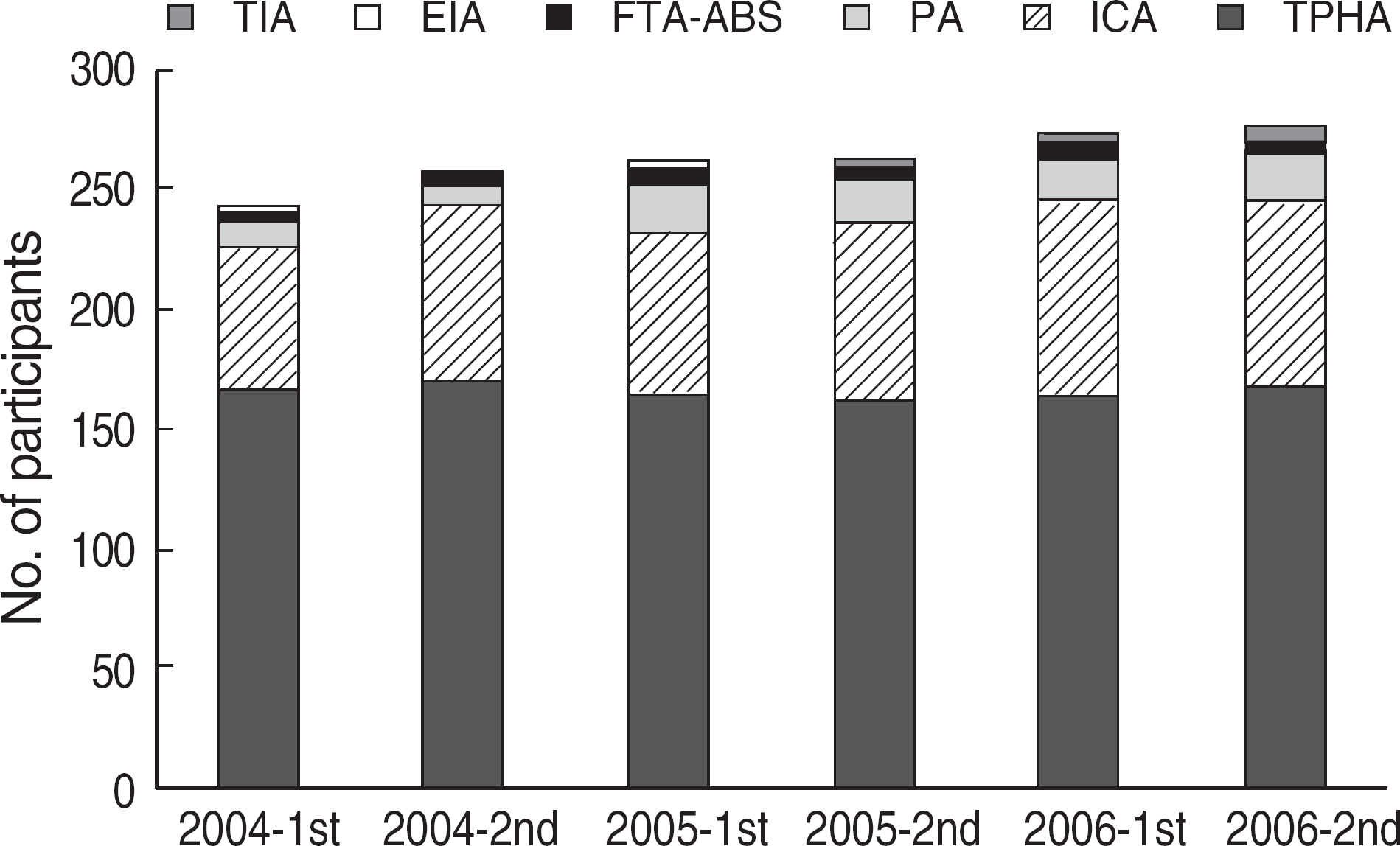 | Fig. 4.Distribution of the methods of treponemal tests used in Korea (2004-2006). Numbers of the participants who used each method were shown in each survey. Abbreviations: No., number; TPHA, Treponema pallidum hemag-glutination assay; ICA, immunochromatography assay; PA, particle agglutination; FTA-ABS, fluorescent treponemal antibody absorption test; EIA, enzyme immunoassay; TIA, turbidoimmunoassay. |
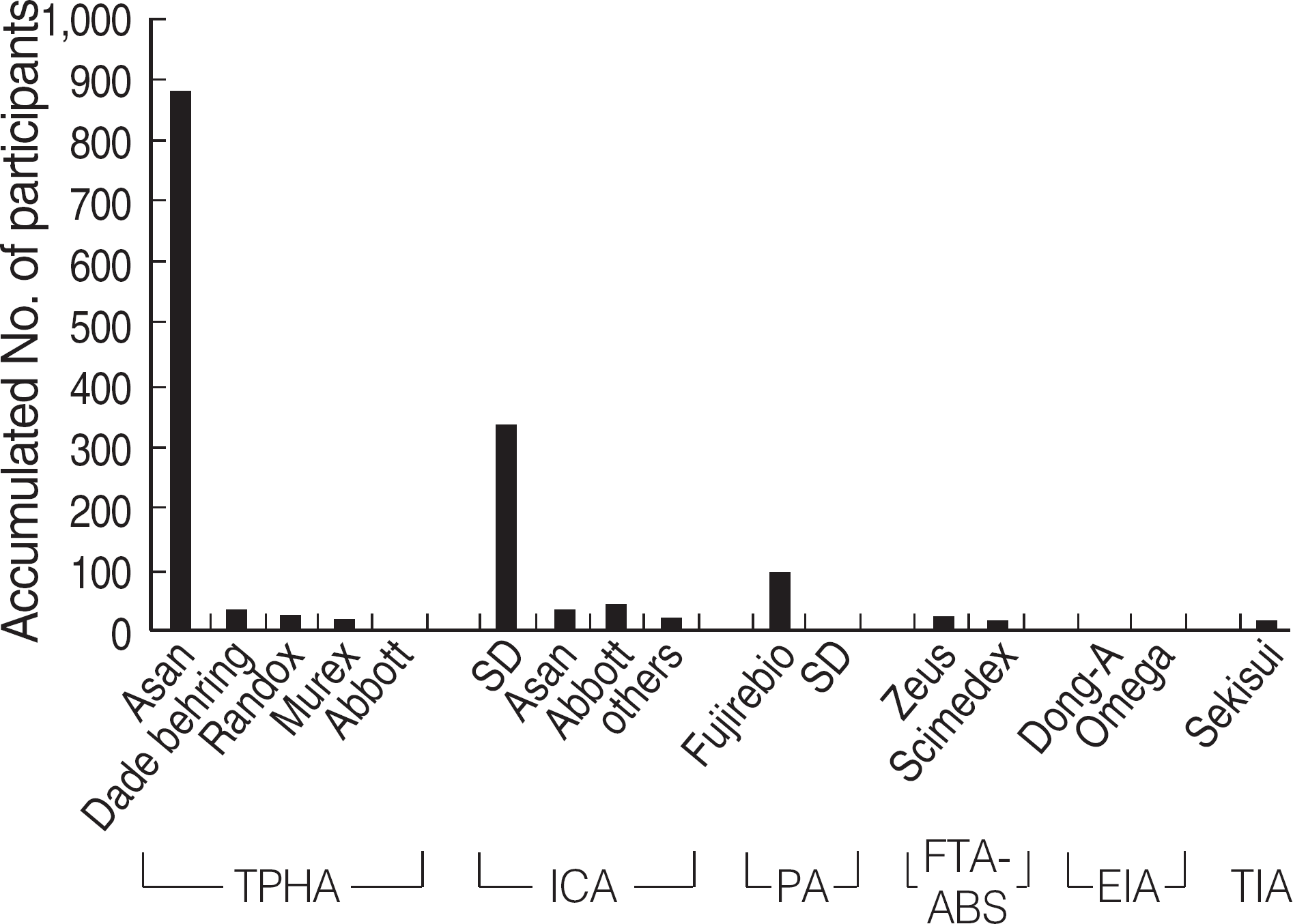 | Fig. 5.Distribution of the manufacturers of TPHA, ICA, FTA-ABS, EIA, and TIA in Korea (2004-2006). Accumulated numbers of the participants who used each method and kit during the 3 yrs of surveys (2004-2006) were shown. Abbreviation: See Fig. 4. |
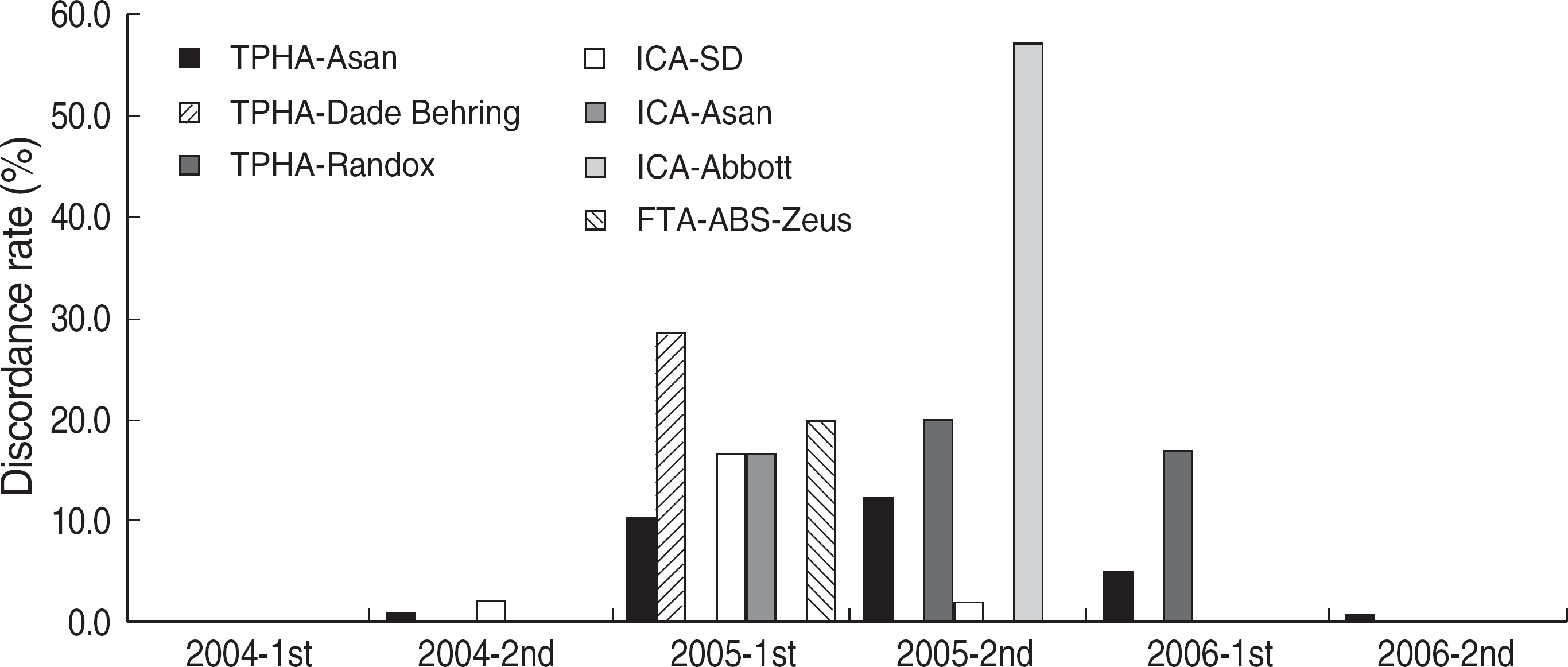 | Fig. 6.Discordance rates of treponemal tests in Korea (2004-2006). Discordance rate was calculated as a number of participants with discordant results divided by a number of the total participants. The discordant result was defined as a result different from the consensus (more than 80% of participants). Abbreviations: TPHA, Treponema pallidum hemagglutination assay; ICA, immunochromatography assay; FTA-ABS, fluorescent treponemal antibody absorption test. |
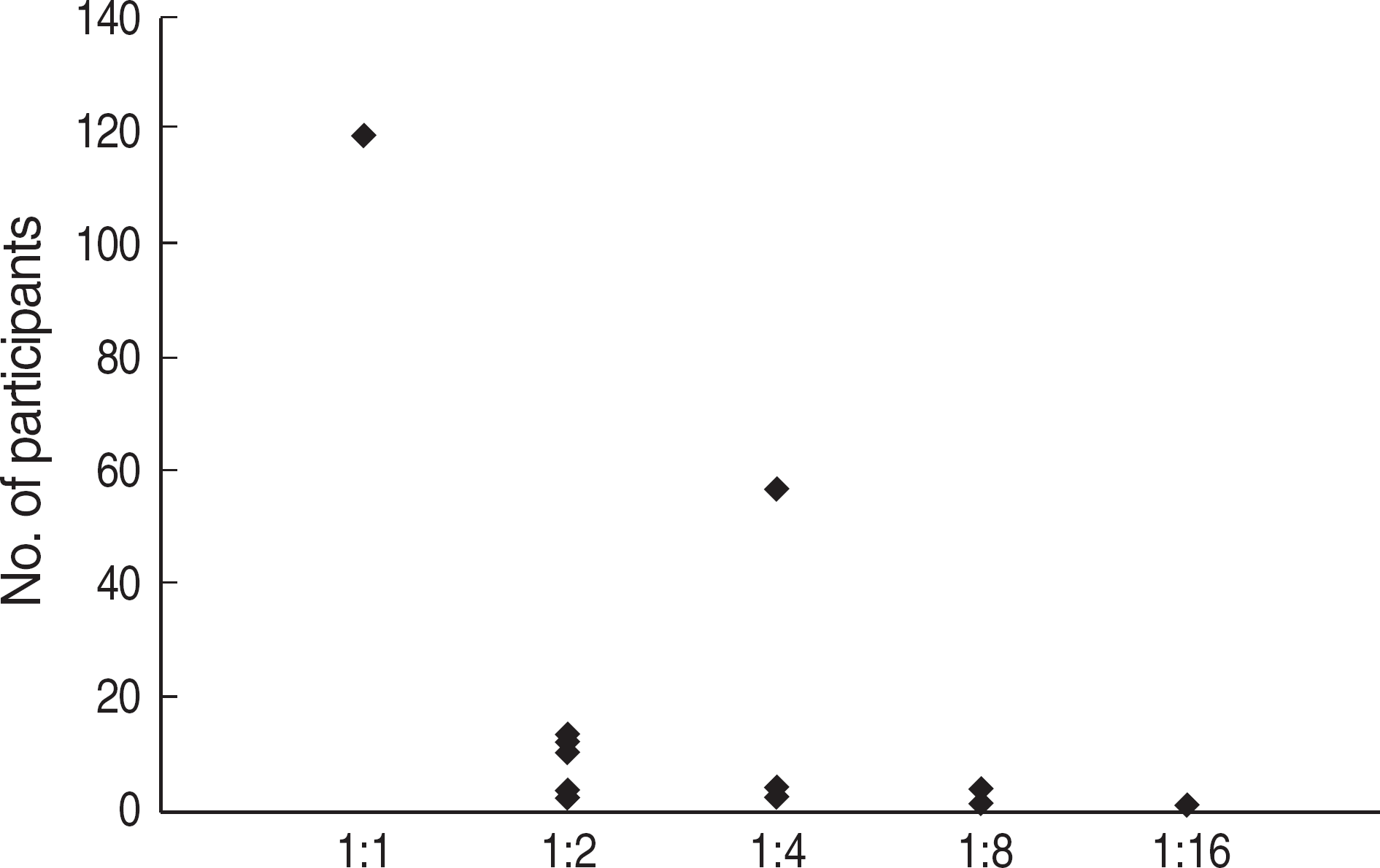 | Fig. 7.A number of participants with discordant results according to the RPR titer of quality assessment materials in CAP survey (2005-2006). Abbreviation: No., number. |
Table 1.
| CAP ('05-'06) | KAQACL ('04-'06) | |
|---|---|---|
| VDRL | 0.1% | 3.6% |
| RPR | 0.3% | 2.2% |
| TPHA/TP-PA/PK-TP | 0.1% | 4.2%∗ |
| FTA-ABS | 0.1% | 2.7% |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download