Abstract
Background
Free light chain (FLC) is widely used to evaluate B-cell proliferative diseases. Herein, we estimated the clinical usefulness of serum FLC in multiple myeloma (MM).
Methods
Fifty-one patients were enrolled. We performed FLC analysis, protein electrophoresis (PEP), and immunofixation electrophoresis (IFE). FLC was measured using Toshiba 200 FR Neo with FREELITE™, and kappa/lambda (κ/λ) ratio was calculated. We compared these parameters in 41 patients with increased FLC before and after bortezomib treatment. Complete response (CR) was defined as the disappearance of monoclonal (M) protein in serum and/or urine as measured by IFE. Partial response (PR) was defined as ≥50% reduction of serum M protein. Early objective response (EOR) included both CR and PR. Minimal response (MR) was defined as 25-49% reduction of M protein and stable disease (SD) as <25% reduction.
Results
Forty-one (80.4%) of the 51 patients studied revealed increment of FLC and the five patients with no increment revealed an abnormal κ/λratio. Especially, all of the light chain myeloma and non-secretory myeloma showed increased FLC concentrations. Among the patients with EOR, 72.4% (21/29) showed a normal or subnormal FLC concentration after the first cycle of treatment. Otherwise, PEP and IFE normalized in 24.1% (7/29) and 24.1% (7/29), respectively. The ratio of decreased FLC after the first cycle of treatment was significantly different between EOR and other response groups (MR, SD) (90.6% vs 51.8%, P=0.011).
REFERENCES
1.Bradwell AR., Carr-Smith HD., Mead GP., Harvey TC., Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003. 361:489–91.

2.Bradwell AR., Carr-Smith HD., Mead GP., Tang LX., Showell PJ., Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001. 47:673–80.

3.Park IJ., Cho SR., Lee WG. Discrimination of monoclonal gammopathy using immunoassay for free light chains. Korean J Lab Med. 2004. 24:91–5. (박일중, 조성란, 이위교. 단클론성 감마글로불린병증 감별을위한유리형경쇄측정. 대한진단검사의학회지 2004;24: 91-5.).
4.Kang SY., Suh JT., Lee HJ., Yoon HJ., Lee WI. Establishment of serum reference range for free light chains and its clinical usefulness in multiple myeloma. Korean J Lab Med. 2004. 24:273–8. (강소영, 서진태, 이희주, 윤휘중, 이우인. 혈청유리형경쇄참고치설정과다발성골수종환자들에서의임상적의의. 대한진단검사의학회지 2004;24: 273-8.).
5.Katzmann JA., Clark RJ., Abraham RS., Bryant S., Lymp JF., Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002. 48:1437–44.
6.Smith A, Wisloff F, Samson D. UK Myeloma Forum, Nordic Myeloma Study Group, British Committee for Standards in Hematology. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006. 132:410–51.
7.Min CK., Lee MJ., Eom KS., Lee S., Lee JW., Min WS, et al. Bortezomib in combination with conventional chemotherapeutic agents for multiple myeloma compared with bortezomib alone. Jpn J Clin Oncol. 2007. 37:961–8.

8.Mead GP., Carr-Smith HD., Drayson MT., Morgan GJ., Child JA., Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004. 126:348–54.

9.Drayson M., Tang LX., Drew R., Mead GP., Carr-Smith H., Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001. 97:2900–2.

10.Epstein WV., Tan M. Increase of L-chain proteins in the sera of patients with systemic lupus erythematosus and the synovial fluids of patients with peripheral rheumatoid arthritis. Arthritis Rheum. 1966. 9:713–9.

11.Hopper JE., Sequeira W., Martellotto J., Papagiannes E., Perna L., Skosey JL. Clinical relapse in systemic lupus erythematosus: correlation with antecedent elevation of urinary free light-chain immunoglobulin. J Clin Immunol. 1989. 9:338–50.

12.Groop L., Makipernaa A., Stenman S., DeFronzo RA., Teppo AM. Urinary excretion of kappa light chains in patients with diabetes mellitus. Kidney Int. 1990. 37:1120–5.

13.Solling K., Solling J., Romer FK. Free light chains of immunoglobulins in serum from patients with rheumatoid arthritis, sarcoidosis, chronic infections and pulmonary cancer. Acta Med Scand. 1981. 209:473–7.
14.Jaskowski TD., Litwin CM., Hill HR. Detection of kappa and lambda light chain monoclonal proteins in human serum: automated immunoassay versus immunofixation electrophoresis. Clin Vaccine Immunol. 2006. 2:277–80.
Fig. 1.
Kinetics of immunoglobulin and free light chain (FLC). FLC with early objective response group normalized rapidly while immunoglobulin decreased slowly.
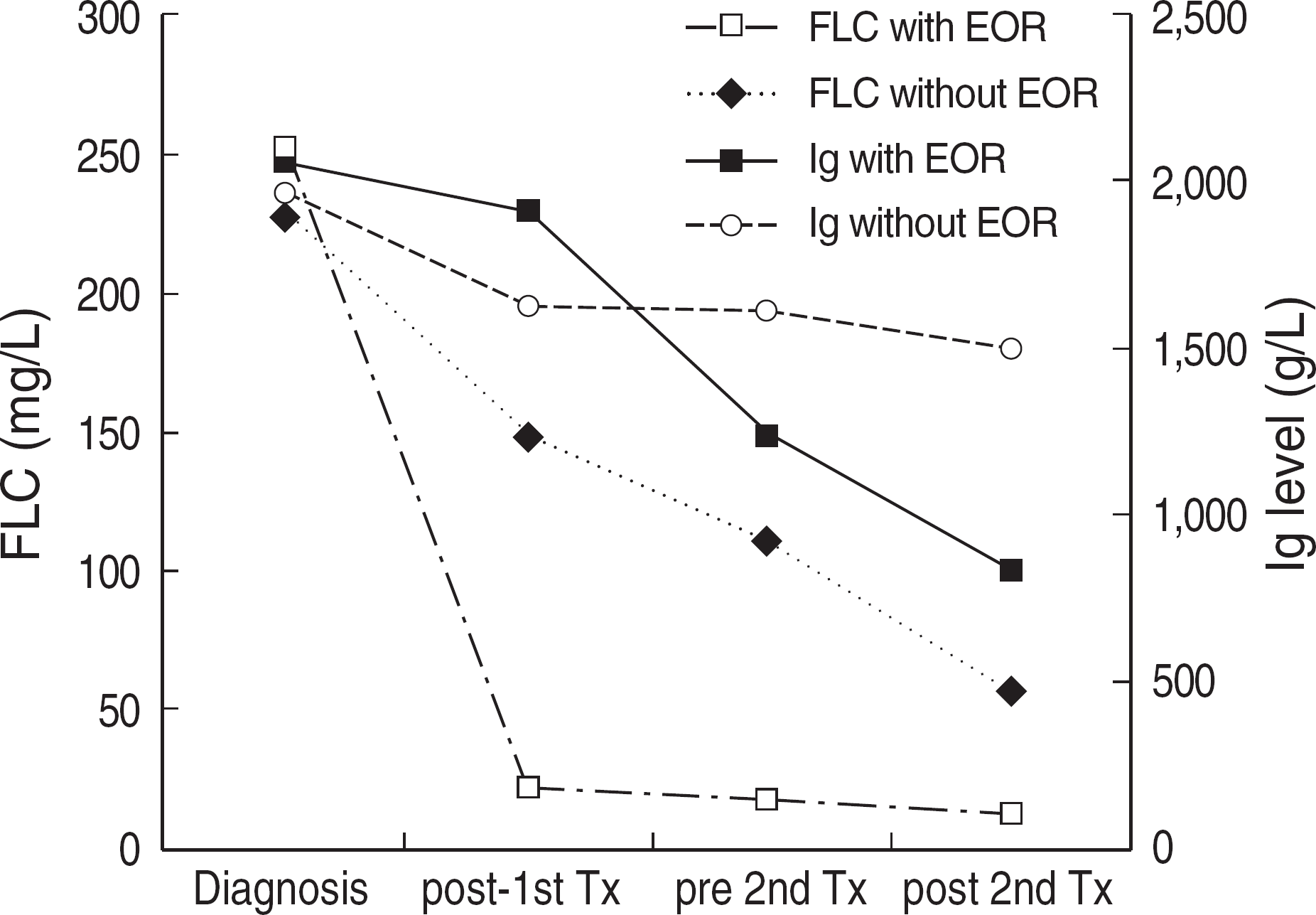
Abbreviations: FLC, free light chain; EOR, early objective response; Ig, immunoglobulin; Tx, treatment
Table 1.
Summary of patients with abnormal serum free light chain at diagnosis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download