Abstract
Background
Hepatitis B virus (HBV) DNA quantification is necessary for starting and monitoring of antiviral therapy in patients with chronic hepatitis B. This study was intended to assess the clinical performance of Abbott RealTime HBV Quantification kit (Abbott Laboratories, USA).
Methods
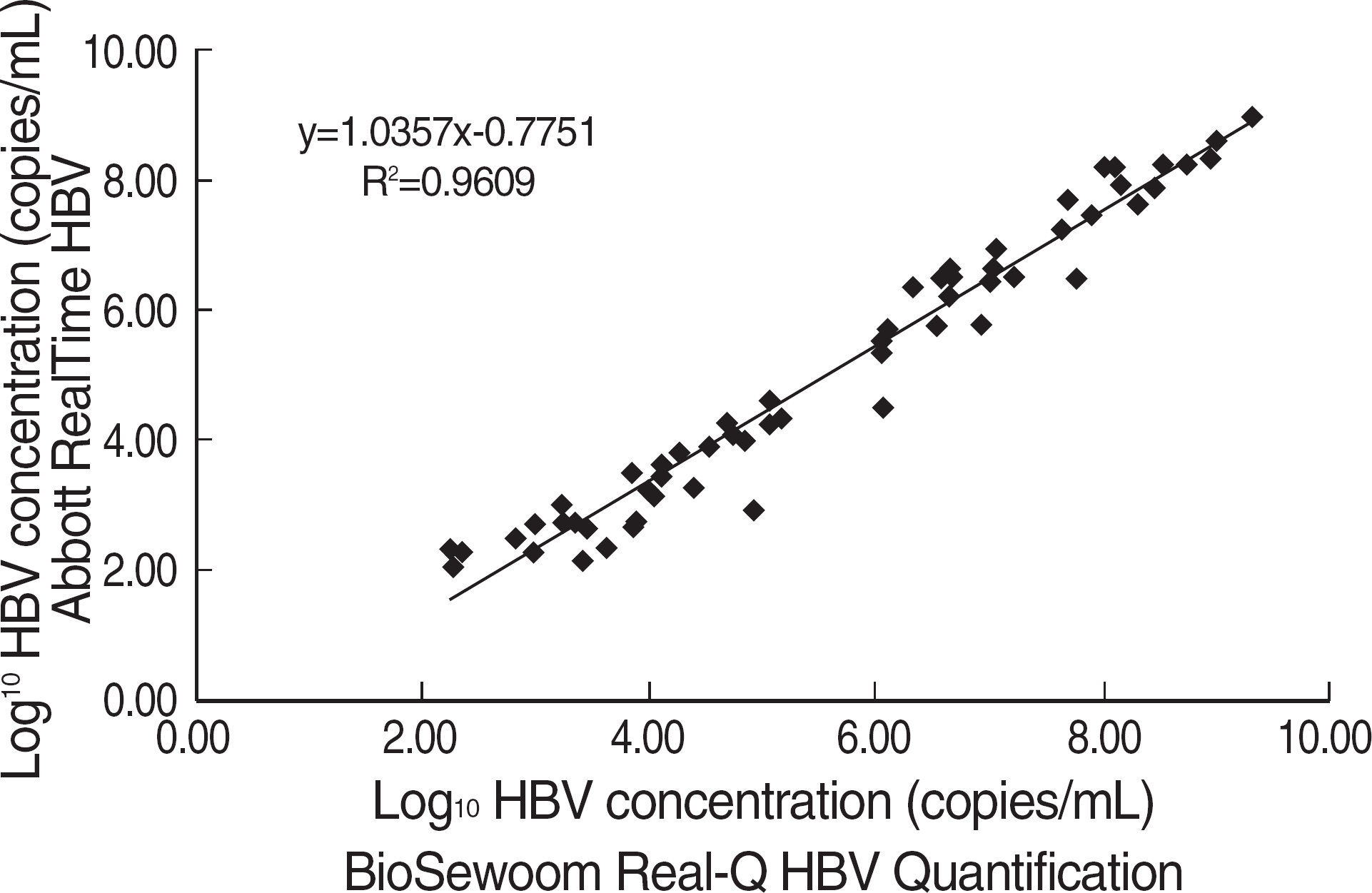
The performance was evaluated in terms of precision, linearity, detection sensitivity, cross-reactivity, and carry-over. A correlation with the Real-Q HBV Quantification kit (BioSewoom Inc., Korea) was also examined using serum samples from 64 patients diagnosed with chronic hepatitis B and underwent lamivudine therapy in Asan Medical Center. We verified the trueness of the system by comparing the outputs with the assigned values of the BBI panel (BBI Diagnostics, USA).
Results
Within-run and between-run coefficients of variation (CV) were 3.56-4.71% and 3.03-4.98%, respectively. Linearity was manifested ranging from 53 to 109 copies/mL and the detection sensitivity was verified to be 51 copies/mL. None of hepatitis C virus showed cross-reactivity. No cross-contamination occurred when negative and positive samples were alternatively placed in a row. It showed a good correlation with the Real-Q HBV (r2=0.9609) and the test results for the BBI panel were also well agreed to the assigned values (r2=0.9933).
REFERENCES
1.Di Bisceglie A., Lai CL., Gane E., Chen YC., Thongsawat S., Wang Y, et al. Telbivudine globe trial: maximal early HBV suppression is predictive of optimal two-year efficacy in nucleoside-treated hepatitis B patients. Hepatology. 2006. 44(S1):230–1.
2.Hadziyannis SJ., Tassopoulos NC., Heathcote EJ., Chang TT., Kitis G., Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006. 131:1743–51.

3.Yuen MF., Sablon E., Hui CK., Yuan HJ., Decraemer H., Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001. 34:785–91.

4.Chen CJ., Yang HI., Su J., Jen CL., You SL., Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006. 295:65–73.

5.Kim SH., Ki CS., Park HK., Cho HJ., Kim JW., Park SS, et al. Annual report on external quality assessment in diagnostic genetics in Korea (2006). J Lab Med Qual Assur. 2007. 29:153–74. (김선희, 기창석, 박현경, 조현정, 김종원, 박성섭등. 진단유전학분과신빙도조사결과보고(2006). 임상검사와정도관리 2007;29: 153-74.).
6.Konnick EQ Erali M., Ashwood ER., Hillyard DR. Evaluation of the COBAS amplicor HBV monitor assay and comparison with the ultrasensitive HBV hybrid capture 2 assay for quantification of hepatitis B virus DNA. J Clin Microbiol. 2005. 43:596–603.

7.Pawlotsky JM., Bastie A., Hezode C., Lonjon I., Darthuy F., Remire J, et al. Routine detection and quantification of hepatitis B virus DNA in clinical laboratories: performance of three commercial assays. J Virol Methods. 2000. 85:11–21.

8.Lee HC., Suh DJ., Ryu SH., Kim H., Shin JW., Lim YS, et al. Quantitative polymerase chain reaction assay for serum hepatitis B virus DNA as a predictive factor for post-treatment relapse after lamivudine induced hepatitis B e antigen loss or seroconversion. Gut. 2003. 52:1779–83.

10.Mackay IM. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004. 10:190–212.

11.Holland PM., Abramson RD., Watson R., Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5′—-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA. 1991. 88:7276–80.

12.Lee LG., Connell CR., Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993. 21:3761–6.
14.Hwang SH., Cha CH., Kim YL., Kwon OJ., Oh HB. Performance evaluation of Real-Q HBV Quantification Kit for HBV DNA by real-time PCR. Korean J Lab Med. 2006. 26:442–8. (황상현, 차충환, 김유리, 권오중, 오흥범. 실시간 정량적 중합효소연쇄반응을 이용한 Real-Q HBV Quantification Kit의성능평가. 대한진단검사의학회지 2006;26: 442-8.).

15.Dienstag JL., Schiff ER., Wright TL., Perrillo RP., Hann HW., Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999. 341:1256–63.

16.Lai CL., Chien RN., Leung NW., Chang TT., Guan R., Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998. 339:61–8.
17.Schalm SW., Heathcote J., Cianciara J., Farrell G., Sherman M., Willems B, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000. 46:562–8.

18.Chang TT., Lai CL., Chien RN., Guan R., Lim SG., Lee CM, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004. 19:1276–82.

19.Lok AS., Lai CL., Leung N., Yao GB., Cui ZY., Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003. 125:1714–22.

20.Fung SK., Chae HB., Fontana RJ., Conjeevaram H., Marrero J., Oberhelman K, et al. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006. 44:283–90.

21.Lee YS., Suh DJ., Lim YS., Jung SW., Kim KM., Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006. 43:1385–91.

22.Klein D. Quantification using real-time PCR technology: applications and limitations. Trends Mol Med. 2002. 8:257–60.

Fig. 1.
The linearity range of Abbott RealTime HBV Quantification kit. Claimed linearity was verified with a result ranging from 53 to 109 copies/mL.
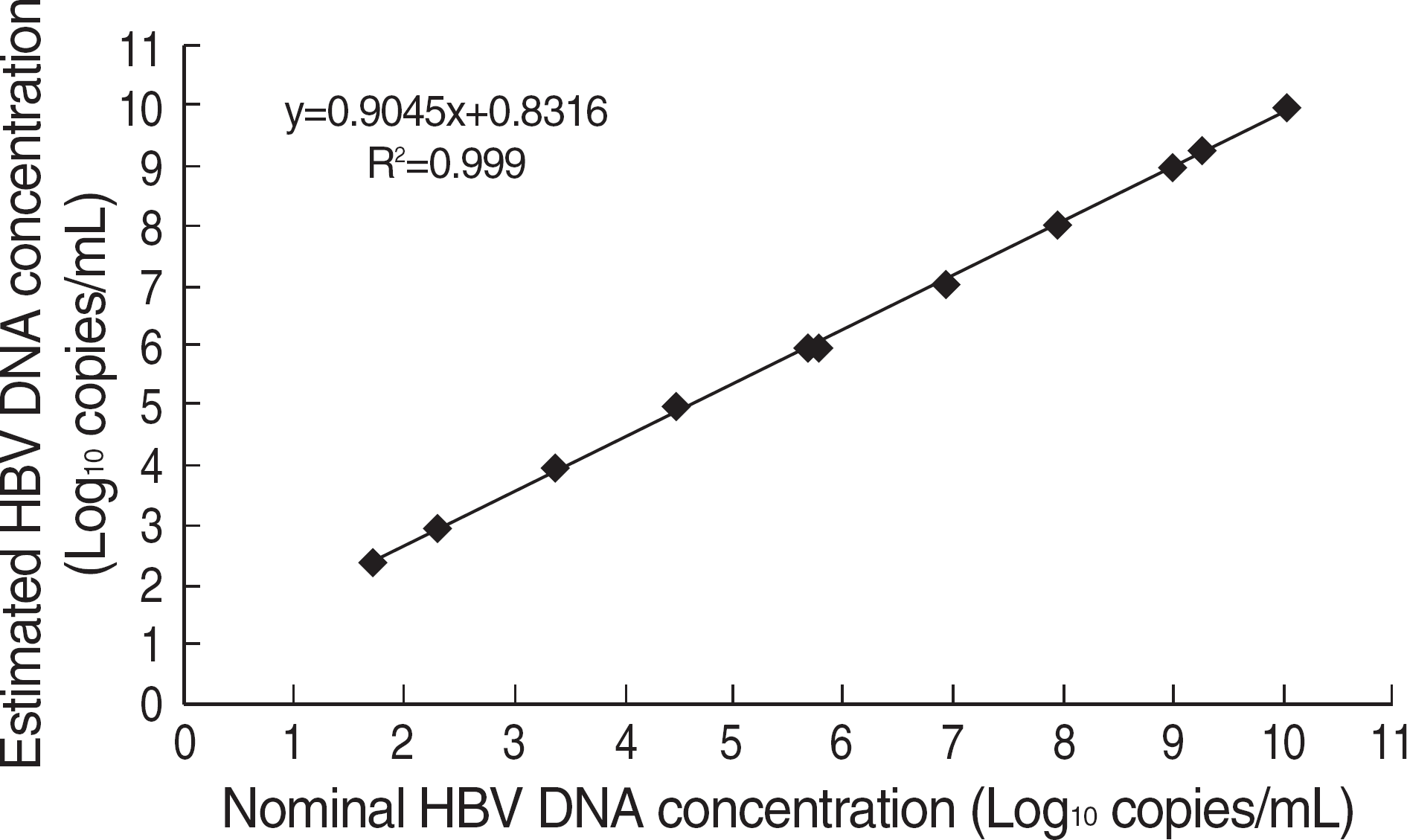
Fig. 2.
Correlation of test results between BBI control target value and Abbott RealTime HBV Quantification kit.
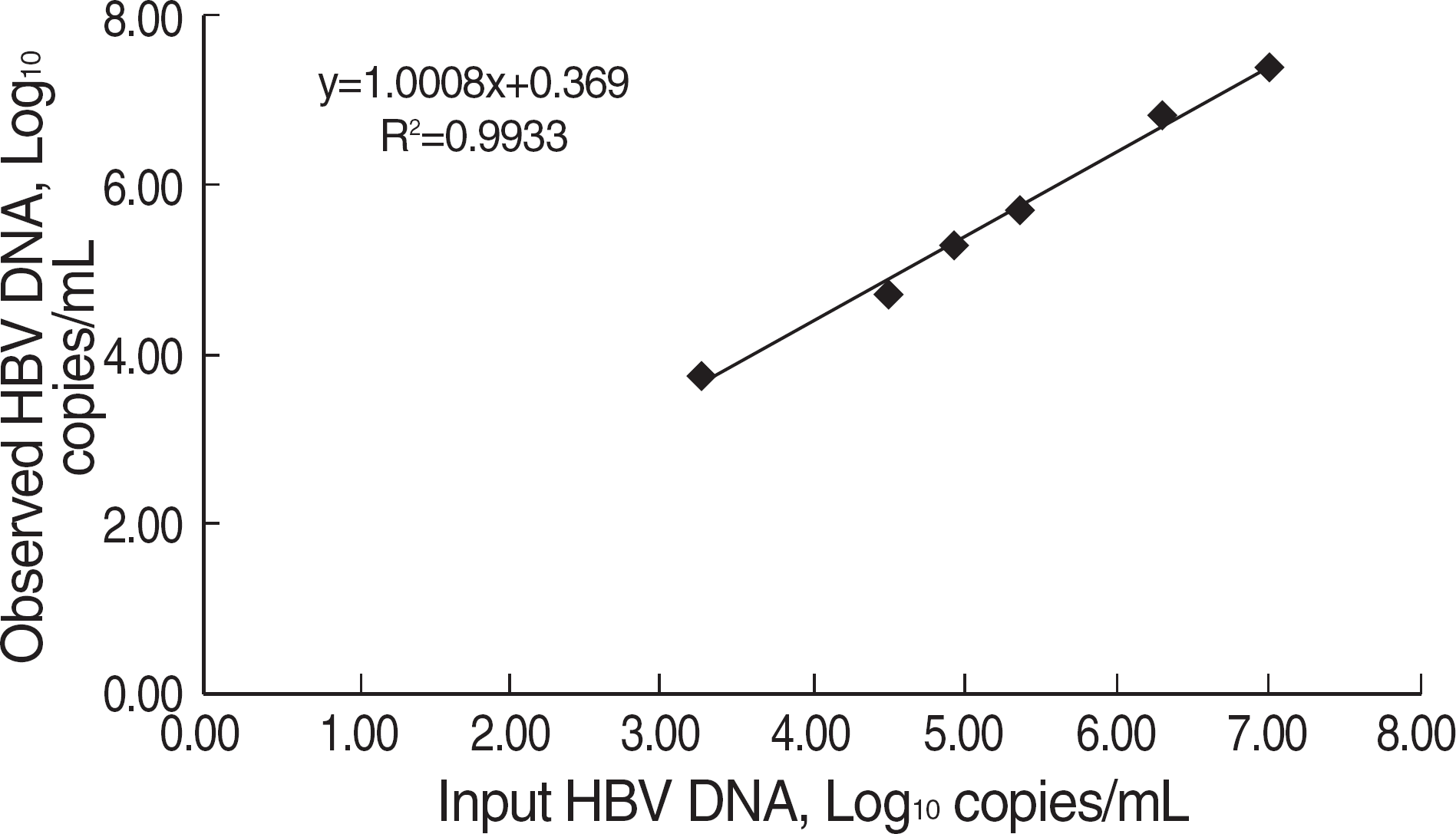
Table 1.
Detection limit of Abbott RealTime HBV Quantification kit
| Input HBV (Copies/mL) | Result | ||
|---|---|---|---|
| Replicates | Positive reaction | % | |
| 270 | 10 | 10 | 100 |
| 135 | 4 | 4 | 100 |
| 90 | 7 | 7 | 100 |
| 67.5 | 10 | 10 | 100 |
| 33.75 | 9 | 9 | 100 |
Table 2.
Carry-over tests of Abbott RealTime HBV Quantification kit




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download