Abstract
Background
Direct antigen test (DAT) and culture are primary tests to diagnose infections by respiratory viruses, but are mainly available for the traditional viral pathogens such as respiratory syncytial virus (RSV), influenza virus, parainfluenza virus (PIV), and adenovirus in clinical laboratories. The objective of this study was to evaluate a multiplex reverse transcriptase-PCR method using Seeplex™ RV Detection kit (Seegene, Korea) for the detection of rhinovirus, coronavirus, and human metapneumovirus (hMPV).
Methods
From January to May 2007, nasopharyngeal aspirates (NPAs) from pediatric patients negative for culture and DAT of traditional viral pathogens were tested with Seeplex™. All the amplicons were directly sequenced and homology of the sequences was searched in the National Center for Biotechnology Information (NCBI) database. Patients’ medical records were reviewed for clinical and demographic features.
Results
Forty-seven (26.4%) of 178 NPAs were positive: 18 rhinovirus, 15 hMPV, 4 RSV A, 3 coronavirus OC43, 3 influenza virus A, 2 adenovirus, 1 coronavirus NL63, and 1 RSV B. Based on maximum identity, each of the sequences indicating rhinovirus, hMPV, and coronavirus OC43 matched to the corresponding viruses with homology of 94-98%, 96-99%, and 98-100%, respectively. Seeplex™-positive patients were 0-11 yr old with a male:female ratio of 1.5:1. Clinical diagnoses included 9 pneumonia, 6 bronchiolitis, 2 cold, 1 asthma exacerbation for rhinovirus; 10 pneumonia, 4 bronchiolitis, and 1 clinical sepsis for hPMV; and 1 pneumonia, 2 croup, and 1 cold for coronavirus.
Go to : 
REFERENCES
1.Chretien J., Holland W., Macklem P., Murray J., Woolcock A. Acute respiratory infections in children. A global public-health problem. N Engl J Med. 1984. 310:982–4.
3.Forbes BA, Sahm DF, editors. Bailey and Scott's diagnostic microbiology. 12th ed.St. Louis: Mosby Elsevier;2007. p. 798–813.
4.Li H., McCormac MA., Estes RW., Sefers SE., Dare RK., Chappell JD, et al. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007. 45:2105–9.

5.Henrickson KJ. Cost-effective use of rapid diagnostic techniques in the treatment and prevention of viral respiratory infections. Pediatr Ann. 2005. 34:24–31.

6.Denny FW., Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986. 108:635–46.

7.Kang JO., Kim EC., Lee KM., Lee NY., Lee CK. Surveillance for respiratory virus testing situation in Korea and epidemiology for the respiratory viruses detected in 5 university hospitals. Korean J Clin Microbiol. 2007. 10:102–8. (강정옥, 김의종, 이규만, 이남용, 이창규. 국내 의료기관의 호흡기 바이러스 검사현황 및 5개 대학병원에서 검출된호흡기바이러스역학. 대한임상미생물학회지 2007;10: 102-8.).
8.Kim SH., Huh JH., Bae SY., Kim JS., Yoon SY., Lim CS, et al. Epidemiology of respiratory viral infection in 2004-2006. Korean J Lab Med. 2006. 26:351–7. (김선형, 허지훈, 배숙영, 김장수, 윤수영, 임채승등. 2004-2006년의 호흡기 바이러스 감염의 역학. 대한진단검사의학회지 2006;26: 351-7.).

9.Williams JV., Harris PA., Tollefson SJ., Halburnt-Rush LL., Pingsterhaus JM., Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004. 350:443–50.

10.Choi EH., Lee HJ., Kim SJ., Eun BW., Kim NH., Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006. 43:585–92.

11.Papadopoulos NG., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A, et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002. 165:1285–9.

12.van der Hoek L., Pyrc K., Jebbink MF., Vermeulen-Oost W., Berkhout RJ., Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. 2004. 10:368–73.

13.Forbes BA, Sahm DF, editors. Bailey and Scott's diagnostic microbiology. 12th ed.St. Louis: Mosby Elsevier;2007. p. 718–76.
14.Espy MJ., Uhl JR., Sloan LM., Buckwalter SP., Jones MF., Vetter EA, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006. 19:165–256.

15.Lam WY., Yeung AC., Tang JW., Ip M., Chan EW., Hui M, et al. Rapid multiplex nested PCR for detection of respiratory viruses. J Clin Microbiol. 2007. 45:3631–40.

16.Mahony J., Chong S., Merante F., Yaghoubian S., Sinha T., Lisle C, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol. 2007. 45:2965–70.

17.Chun JY., Kim KJ., Hwang IT., Kim YJ., Lee DH., Lee IK, et al. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007. 35:e40.

18.Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004. 5:150–63.

19.Tang YW., Crowe JE Jr. Respiratory syncytial virus and human metapneumovirus. Murray PR, Baron EJ, editors. Manual of clinical microbiology. 9th ed.Washington DC: ASM Press;2007. p. 1361–77.
20.Lee WM., Grindle K., Pappas T., Marshall DJ., Moser MJ., Beaty EL, et al. High-throughput, sensitive, and accurate multiplex PCR-micro-sphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007. 45:2626–34.

21.Kim SR., Ki CS., Lee NY. Rapid identification of 12 respiratory viruses with a multiplex PCR assay. Korean J Lab Med. 2007. 27(S):S526–7.
22.Landry ML. Rhinoviruses. Murray PR, Baron EJ, editors. Manual of clinical microbiology. 9th ed.Washington DC: ASM Press;2007. p. 1405–13.

23.Mahony JB. Coronaviruses. Murray PR, Baron EJ, editors. Manual of clinical microbiology. 9th ed.Washington DC: ASM Press;2007. p. 1414–23.
24.van den Hoogen BG., Osterhaus DM., Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004. 23(S):S25–32.

25.Yoo SJ., Kuak EY., Shin BM. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system. Korean J Lab Med. 2007. 27:420–7. (유수진, 곽은영, 신보문. Dual priming oligonucleotide system을 이용한 다중 역전사 PCR 키트를 통한 12가지 호흡기 바이러스의 검출. 대한진단검사의학회지 2007;27: 420-7.).

26.Krilov L., Pierik L., Keller E., Mahan K., Watson D., Hirsch M, et al. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J Med Virol. 1986. 19:345–52.

27.McMillan JA., Weiner LB., Higgins AM., Macknight K. Rhinovirus infection associated with serious illness among pediatric patients. Pediatr Infect Dis J. 1993. 12:321–5.

28.Kim YK., Lee HJ. Human metapneumovirus-associated lower respiratory tract infections in korean infants and young children. Pediatr Infect Dis J. 2005. 24:1111–2.

29.Yeom HH., Park JS., Jeong DJ., Kim CJ., Kim YB., Lee DH, et al. Human metapneumovirus infection in Korean children. Korean J Pediatr. 2006. 49:401–9. (염희현, 박준수, 정동준, 김창진, 김용배, 이대훈 등. 소아에서 human metapneumovirus 감염. 대한소아과학회지 2006;49: 401-9.).

30.Han TH., Chung JY., Kim SW., Hwang ES. Human Coronavirus-NL63 infections in Korean children, 2004-2006. J Clin Virol. 2007. 38:27–31.

31.Leland DS., Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007. 20:49–78.

Go to : 
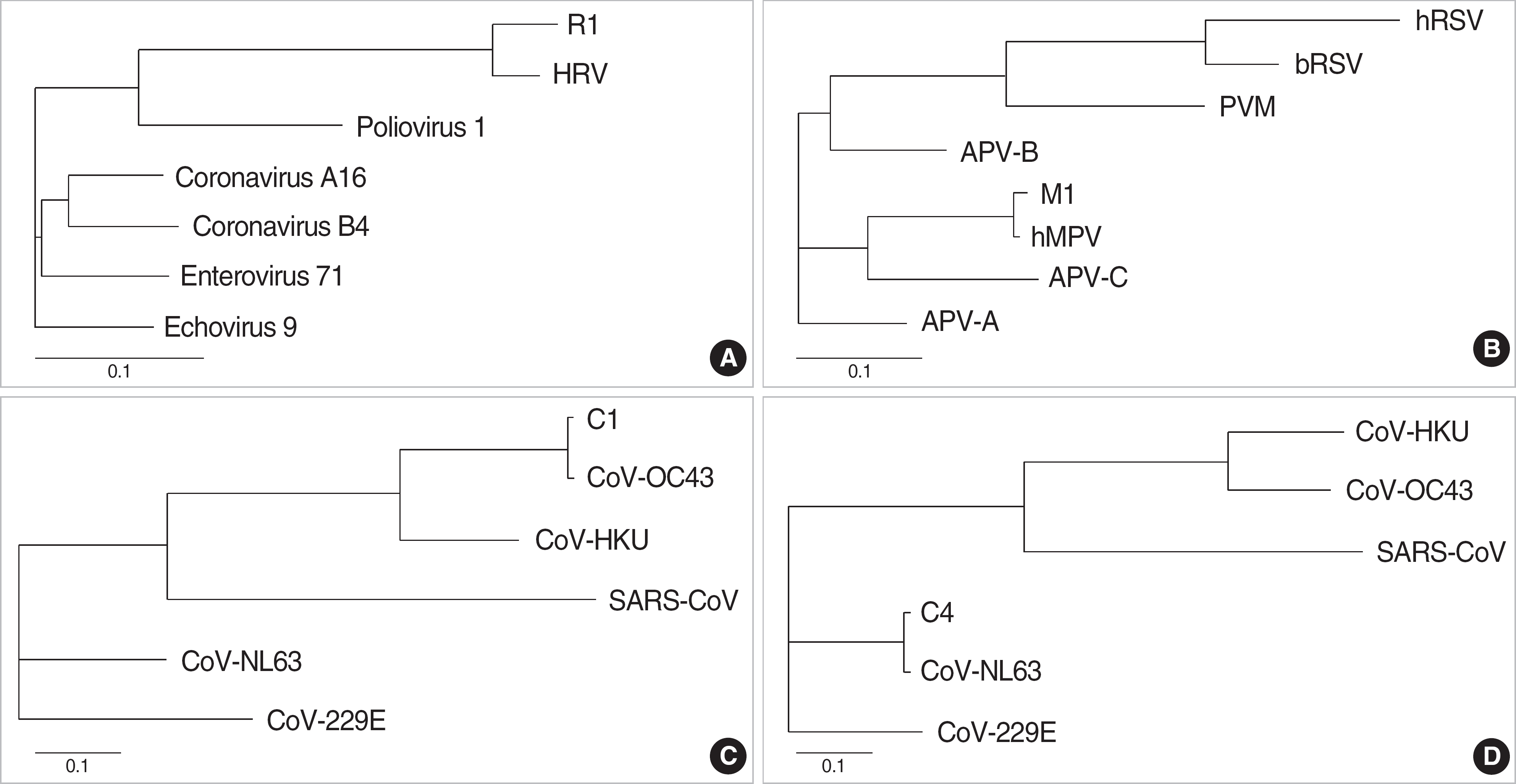 | Fig. 1.Phylogenetic analysis using Neighbour-Joining method showed a close relationship between the isolates of this study (A. Human rhinovirus [HRV] R1, B. Human metapneumovirus [hMPV] M1, C. Coronavirus [CoV] OC43 C1, D. CoV NL63 C4) and archival strains of corresponding viruses. Distances are presented as a number of substitutions per site (a scale of ‘0.1’ means 0.1 nucleotide substitution per site). Abbreviations: hRSV, human respiratory syncytial virus; bRSV, bovine respiratory syncytial virus; PVM, pneumonia virus of mice; APV, avian pneumovirus; SARS, severe acute respiratory syndrome. |
Table 1.
Clinical diagnoses of 47 patients positive for rhinovirus, human metapneumovirus, coronavirus, and other viruses by multiplex reverse transcriptase PCR
| Respiratory viruses | Total | N of patients | |||||
|---|---|---|---|---|---|---|---|
| Pneumonia | Bronchiolitis | Croup | Asthma exacerbation | Clinical sepsis | Common cold | ||
| HRV | 18 | 9 | 6 | 0 | 1 | 0 | 2 |
| hMPV | 15 | 10 | 4 | 0 | 0 | 1 | 0 |
| CoV OC43 | 3 | 1 | 0 | 1 | 0 | 0 | 1 |
| CoV NL63 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Others∗ | 10 | 3 | 5 | 0 | 0 | 2 | 0 |
| Total | 47 | 23 | 15 | 2 | 1 | 3 | 3 |
Table 2.
Similarities between the sequences of the respiratory viral isolates in this study and the archival sequences from the GenBank




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download