Abstract
Background
The aberrant, leukemia-associated antigen expression patterns allow us to discriminate leukemic blasts from normal precursor cells. Our major goal was to determine a guideline for the detection of minimal residual disease using CD20+/CD34+ and myeloid Ag+/CD19+ combination in the bone marrow of acute leukemia in complete remission (CR) after chemotherapy.
Methods
Bone marrow samples from 117 patients with acute leukemia in complete remission after chemotherapy and from 22 healthy controls were immunophenotyped by triple staining and measured by flow cytometry.
Results
The CD20+/CD34+ cells in the large lymphocyte gate (R1) ranged from 0% to 3.24% (0.8±0.82%, P=0.000) in CD20+/CD34+ B-lineage ALL CR (N=31), from 0.03% to 4.2% (0.7±0.83 %, P=0.000) in CD20-/CD34- B-lineage ALL CR (N=66), from 0.1% to 0.96% (0.45±0.32%, P=0.016) in T-ALL CR (N=10), and from 0.02% to 0.48% (0.18±0.15%, P=0.776) in AML CR (N=10). The CD13,33+/CD19+ cells in R1 gate ranged from 0% to 2.69% (0.37±0.48%, P<0.001) in CD13,33+/CD19+ B-lineage ALL CR (N=31), from 0% to 1.8% (0.31±0.28%, P<0.001) in CD13,33-/CD19+ B-lineage ALL CR (N=65), from 0.02% to 0.64% (0.29±0.22%, P=0.071) in T-ALL CR (N=9), and from 0% to 0.17% (0.07±0.09%, P=0.341) in AML CR (N=3).
Conclusions
Using an immunophenotypic method for the detection of early relapse or minimal residual disease of B-lineage ALL bone marrow in CR after chemotherapy, different cutoff values should be applied according to antigen combination and gating. When the proportion of aberrant antigen combination was less than 5% in large lymphocyte gate, the results should be interpreted with caution.
REFERENCES
1.Van Wering ER., van der Linden-Schrever BE., Szczepanski T., Willemse MJ., Baars EA., van Wijngaarde-Schmitz HM, et al. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: implications for monitoring of minimal residual disease. Br J Haematol. 2000. 110:139–46.

2.Silverman LB., Gelber RD., Dalton VK., Asselin BL., Barr RD., Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001. 97:1211–8.

3.Schrappe M., Reiter A., Ludwig WD., Harbott J., Zimmermann M., Hiddemann W, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000. 95:3310–22.
4.Caldwell CW., Poje E., Helikson MA. B-cell precursors in normal pediatric bone marrow. Am J Clin Pathol. 1991. 95:816–23.

5.Muehleck SD., McKenna RW., Gale PF., Brunning RD. Terminal deoxynucleotidyl transferase (TdT)-positive cells in bone marrow in the absence of hematologic malignancy. Am J Clin Pathol. 1983. 79:277–84.
6.Longacre TA., Foucar K., Crago S., Chen IM., Griffith B., Dressler L, et al. Hematogones: a multiparameter analysis of bone marrow precursor cells. Blood. 1989. 73:543–52.

7.Davis RE., Longacre TA., Cornbleet PJ. Hematogones in the bone marrow of adults. Immunophenotypic features, clinical settings, and differential diagnosis. Am J Clin Pathol. 1994. 102:202–11.
8.Lucio P., Gaipa G., van Lochem EG., van Wering ER., Porwit-Mac-Donald A., Faria T, et al. BIOMED-I concerted action report: flow cytometric immunophenotyping of precursor B-ALL with standardized triple-stainings. BIOMED-1 Concerted Action Investigation of Minimal Residual Disease in Acute Leukemia: International Standardization and Clinical Evaluation. Leukemia. 2001. 15:1185–92.
9.Ciudad J., Orfao A., Vidriales B., Macedo A., Martinez A., Gonzalez M, et al. Immunophenotypic analysis of CD19+ precursors in normal human adult bone marrow: implications for minimal residual disease detection. Haematologica. 1998. 83:1069–75.
10.Vogel P., Bassen FA. Sternal marrow of children in normal and pathologic states. Am J Dis Child. 1939. 57:245–68.
11.Mckenna RW., Asplund SL., Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4-color flow cytometry. Leuk Lymphoma. 2004. 45:277–85.

12.Van Dongen JJ., Szczepanski T., de Bruijn MA., van den Beemd MW., de Bruin-Versteeg S., Wijkhuijs JM, et al. Detection of minimal residual disease in acute leukemia patients. Cytokines Mol Ther. 1996. 2:121–33.
13.Ciudad J., San Miguel JF., Lopez-Berges MC., Garcia Marcos MA., Gonzalez M., Vazquez L, et al. Detection of abnormalities in B-cell differentiation pattern is a useful tool to predict relapse in precursor-B-ALL. Br J Haematol. 1999. 104:695–705.

14.Dworzak MN., Fritsch G., Fleischer C., Printz D., Froschl G., Buchinger P, et al. Comparative phenotype mapping of normal vs malignant pediatric B-lymphopoiesis unveils leukemia-associated aberrations. Exp Hematol. 1998. 26:305–13.
15.Cap J., Babusikova O., Kaiserova E., Jamarik M. Expression of CD10, CD19 and CD34 markers in bone marrow samples of children with precursor B cell acute lymphoblastic leukemia in clinical and hematological remission. Neoplasma. 1998. 45:231–6.
16.Hurwitz CA., Loken MR., Graham ML., Karp JE., Borowitz MJ., Pullen DJ, et al. Asynchronous antigen expression in B lineage acute lymphoblastic leukemia. Blood. 1988. 72:299–307.

17.Dworzak MN., Fritsch G., Fleischer C., Printz D., Froschl G., Buchinger P, et al. Multiparameter phenotype mapping of normal and post-chemotherapy B lymphopoiesis in pediatric bone marrow. Leukemia. 1997. 11:1266–73.

18.Ciudad J., San Miguel JF., Lopez-Berges MC., Garcia Marcos MA., Gonzalez M., Vazquez L, et al. Detection of abonormalities in B-cell differentiation pattern is a useful tool to predict relapse in precursor-B-ALL. Br J Hematol. 1999. 104:695–705.
Fig. 1.
Dot plot scattergrams of bone marrow cells of a B-lineage ALL patient stained with anti-CD20-PE/anti-CD34-PerCP. The large lymphocyte gate (R1) and all nucleated element gate (R2) were set using forward scatter (FSC) and side scatter (SSC) characteristics. The CD20+/CD34+ cells in the R1 gate (upper right quadrant) were 1.26%.
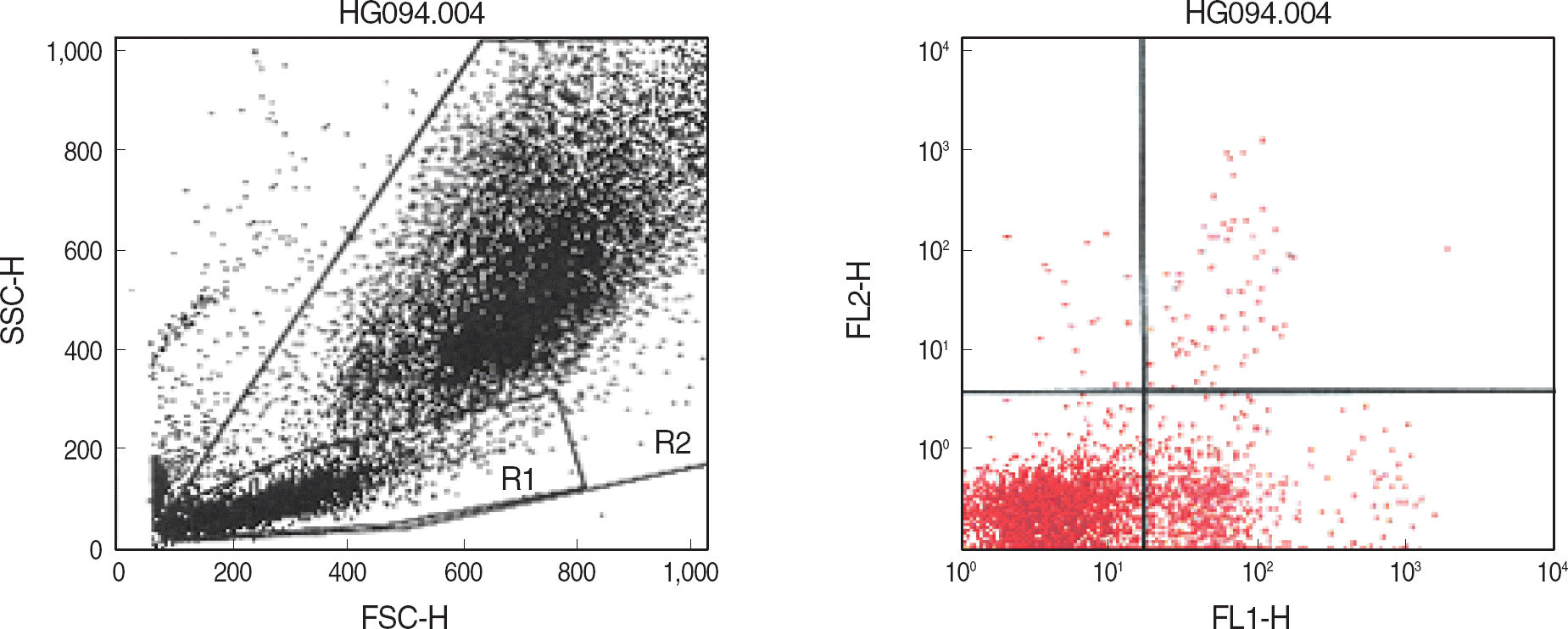
Table 1.
Proportion of CD20+/CD34+ cells in the large lymphocyte gate (R1) and all nucleated element gate (R2) in the bone marrow of acute leukemia patients in complete remission (mean±SD %)
| Gate | Normal control (N=22) | B-ALL, CR (N=97) | T-ALL, CR (N=10) | AML, CR (N=10) | |
|---|---|---|---|---|---|
| Initial Dx CD20,34+ (N=31) | Initial Dx CD20,34- (N=66) | ||||
| R1 | 0.18±0.12 (0-0.47) | 0.8±0.82∗ (0-3.24) | 0.7±0.83∗ (0.03-4.2) | 0.45±0.32∗ (0.1-0.96) | 0.18±0.15 (0.02-0.48) |
| P<0.001 | P<0.001 | P=0.016 | P=0.776 | ||
| R2 | 0.13±0.12 (0-0.3) | 0.17±0.18 (0-0.72) | 0.16±0.17 (0.01-1.76) | 0.17±0.2 (0.01-0.49) | 0.23±0.17 (0.01-0.43) |
| P=0.796 | P=0.548 | P=1.0 | P=0.11 | ||
Table 2.
Proportion of CD13,33+/CD19+ cells in the large lymphocyte gate (R1) and all nucleated element gate (R2) in the bone marrow of acute leukemia patients in complete remission (mean±SD %)
| Gate | Normal control (N=17) | B-ALL, CR (N=96) | T-ALL, CR (N=9) | AML, CR (N=3) | |
|---|---|---|---|---|---|
| CD13,33+ (N=31) | Initial Dx CD13,33- (N=65) | ||||
| R1 | 0.12±0.08 (0-0.29) | 0.37±0.48∗ (0.06-2.69) | 0.31±0.28∗ (0-1.8) | 0.29±0.22 (0.02-0.64) | 0.07±0.09 (0-0.17) |
| P<0.001 | P<0.001 | P=0.071 | P=0.341 | ||
| R2 | 0.42±0.29 (0.01-0.98) | 0.43±0.26 (0.01-0.94) | 0.52±0.34 (0.01-1.76) | 0.49±0.28 (0.01-0.81) | 0.36±0.15 (0.23-0.52) |
| P=0.737 | P=0.101 | P=0.483 | P=0.916 | ||
Table 3.
Proportion of CD15+/CD19+ cells in the large lymphocyte gate (R1) and all nucleated element gate (R2) in the bone marrow of patients with B-lineage ALL complete remission (mean±SD %)
| Gate | Normal control (N=17) | B-ALL, CR (N=11) |
|---|---|---|
| R1 | 1.49±0.98 (0.22-2.9) | 0.38±0.41 (0.01-1.17) |
| P=0.001∗ | ||
| R2 | 1.4±0.64 (0.29-2.41) | 1.39±0.85 (0.27-2.98) |
| P=0.744 |
Table 4.
Proportion of CD11b+/CD19+ cells in the large lymphocyte gate (R1) and all nucleated element gate (R2) in the bone marrow of acute leukemia patients in complete remission (mean±SD %)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download