Abstract
Background
Disseminated intravascular coagulation (DIC) is a syndrome characterized by a systemic activation of coagulation leading to the intravascular deposition of fibrin and the simultaneous consumption of coagulation factors and platelets. Inflammatory cytokines can activate the coagulation system. This study investigated the diagnostic and prognostic usefulness of the plasma level of interleukin-6 (IL-6) and interleukin-10 (IL-10) for predicting DIC.
Methods
The study populations were 15 healthy controls and 81 patients who were clinically suspected of having DIC and were requested to perform DIC battery tests. The presence of overt DIC was defined by the International Society on Thrombosis and Haemostasis Subcommittee cumulative score of 5 or above. The 28 day mortality was used to assess the prognostic outcome. The plasma levels of the cytokines were measured by ELISA.
Results
The plasma levels of IL-6 and IL-10 in patients (N=81) were higher than those of control (N=15). IL-6 and IL-10 levels of overt DIC group (N=31) were 3 times and 1.5 times higher than those, respectively, of non-overt DIC group (N=50). In infection group (N=48), IL-6 and IL-10 levels of overt DIC group (N=18) were 5 times and 3 times higher than those, respectively, of non-overt DIC group (N=30). The diagnostic efficiency of IL-6 (optimal cut off >40.4 pg/mL) and IL-10 (>9.7 pg/mL) for the diagnosis of overt DIC were 67% and 69%, respectively, which were similar to that of D-dimer. Plasma levels of IL-6 and IL-10 were also higher in non-survivors than in survivors. The patients with higher levels of IL-6 and IL-10 showed a poorer prognosis.
Go to : 
References
2. Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001; 86:1327–30.

3. Levi M, de Jonge E, Meijers J. The diagnosis of disseminated intra-vascular coagulation. Blood Rev. 2002; 16:217–23.

4. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000; 148:209–14.

5. Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Inter-feron beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987; 84:7251–5.

6. Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993; 103:565–75.
7. Yang M, Li K. The role of cytokines and transcription factors in megakaryocytopoiesis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002; 10:580–5.
8. Wada H, Tanigawa M, Wakita Y, Nakase T, Minamikawa K, Kaneko T, et al. Increased plasma level of interleukin-6 in disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 1993; 4:583–90.

9. Cassatella MA. The neutrophil: one of the cellular targets of inter-leukin-10. Int J Clin Lab Res. 1998; 28:148–61.

10. Tryzmel J, Miskolci V, Castro-Alcaraz S, Vancurova I, Davidson D. Interleukin-10 inhibits proinflammatory chemokine release by neutrophils of the newborn without suppression of nuclear factor-kappa B. Pediatr Res. 2003; 54:382–6.
11. Chernoff AE, Granowitz EV, Shapiro L, Vannier E, Lonnemann G, Angel JB, et al. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995; 154:5492–9.
12. Wen H, Hogaboam CM, Gauldie J, Kunkel SL. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am J Pathol. 2006; 168:1940–50.

13. Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, et al. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005; 94:975–9.

14. Ng PC, Li G, Chui KM, Chu WC, Li K, Wong RP, et al. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res. 2004; 56:796–803.

15. Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, et al. Increased plasma levels of inter-leukin-6 in sepsis. Blood. 1989; 74:1704–10.
17. Taylor FB Jr, Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991; 33:127–34.
18. Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. 2001; 345:408–16.

19. Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001; 29:S90–4.

20. van Hinsbergh VW, Bauer KA, Kooistra T, Kluft C, Dooijewaard G, Sherman ML, et al. Progress of fibrinolysis during tumor necrosis factor infusions in humans. Concomitant increase in tissue-type plasminogen activator, plasminogen activator inhibitor type-1, and fibrin(ogen) degradation products. Blood. 1990; 76:2284–9.

21. Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003; 35:535–44.

22. Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M, et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997; 17:3399–405.

23. Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, et al. Plasminogen activator inhibitor-1 expression and secretion are stimulated by growth hormone and interleukin-6 in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2006; 253:56–62.

24. Minamikawa K, Wada H, Ohiwa M, Kaneko T, Tsukada T, Kageyama S, et al. Plasma interleukin-6 in patients with disseminated intravascular coagulation. Rinsho Ketsueki. 1992; 33:1797–801.
25. Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991; 147:3815–22.
26. Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994; 153:811–6.
27. Kamimura M, Viedt C, Dalpke A, Rosenfeld ME, Mackman N, Cohen DM, et al. Interleukin-10 suppresses tissue factor expression in lipopolysaccharide-stimulated macrophages via inhibition of Egr-1 and a serum response element/MEK-ERK1/2 pathway. Circ Res. 2005; 97:305–13.

28. Ng PC, Li K, Leung TF, Wong RP, Li G, Chui KM, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006; 52:1181–9.

29. Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt JT, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004; 2:1924–33.
Go to : 
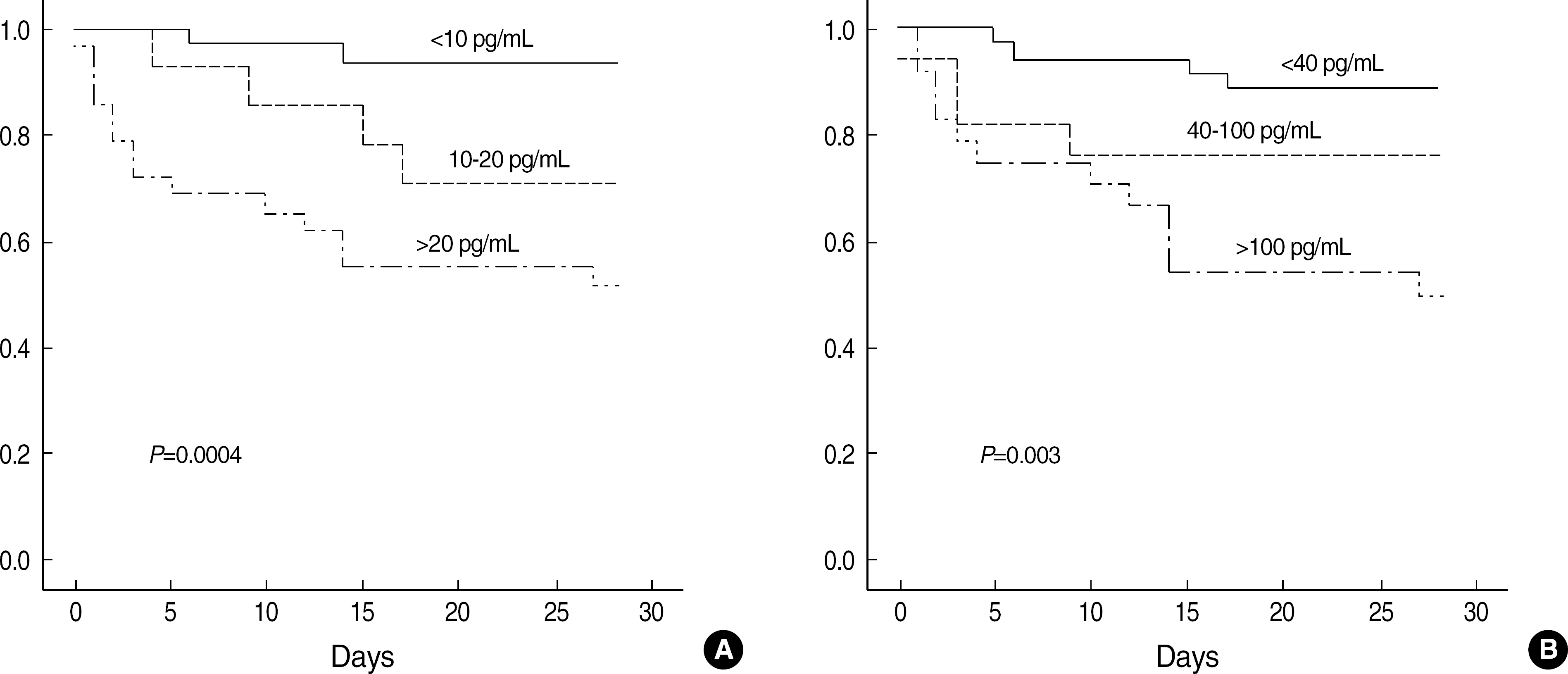 | Fig. 1.Survival curves for patients subdivided by plasma concentrations of IL-6 (A) and IL-10 (B). |
Table 1.
Characteristics of the study population
| Control (N=15) | Patients (N=81) |
Patients |
||
|---|---|---|---|---|
| Overt DIC (N=31) | Non-overt DIC (N=50) | |||
| Age (yr)* | 38 (29–47) | 55 (35–68) | 50 (35–67) | 58 (36–69) |
| Sex of male, N (%) | 9 (60) | 47 (58) | 21 (68) | 26 (52) |
| WBC (×109/L) | 6.07 (4.77–7.41) | 7.39 (3.57–13.54) | 5.24 (2.39–13.48) | 9.3 (5.82–13.65) |
| Hb (g/L)*,§ | 140 (136–150) | 97 (86–114) | 90 (83–104) | 98 (92–117) |
| Platelet (× 10 9 /L)*,‡ | 275 (229–306) | 82 (37–172) | 35 (20–69) | 132 (83–272) |
| Positive FDP, N (%) | 78 (96) | 30 (97) | 48 (96) | |
| D-dimer (μg/mL)‡ | 3.54 (2.39–8.41) | 4.59 (2.70–14.16) | 3.33 (2.10–5.45) | |
| Fibrinogen (mg/dL)‡ | 364 (217–629) | 198 (174–295) | 541 (364–668) | |
| PT (sec)†,‡,‖ | 13.4 (13.2–14.0) | 16.1 (14.0–19.9) | 20.3 (18.2–22.5) | 14.4 (13.0–15.8) |
| aPTT (sec)†,‡,‖ | 38.0 (34.1–40.5) | 47.1 (38.8–55.7) | 52.1 (43.9–60.2) | 42.7 (37.8–54.4) |
| CRP (mg/dL)¶ | 5.73 (1.66–14.55) | 4.86 (1.10–11.44) | 6.36 (1.83–15.91) | |
| IL-6 (pg/mL)*,‡ | 1.00 (1.00–3.48) | 52.10 (12.04–179.25) | 105.50 (50.48–379.6) | 31.83 (6.42–109.80) |
| IL-10 (pg/mL)*,‡ | 2.70 (2.29–11.80) | 10.52 (6.16–23.36) | 13.16 (10.25–46.35) | 8.35 (4.88–20.81) |
Table 2.
Comparison of plasma IL-6 and IL-10 levels in patients with or without infection
|
Infection |
Non-infection |
|||
|---|---|---|---|---|
| Overt DIC (N=18) | Non-overt DIC (N=30) | Overt DIC (N=13) | Non-overt DIC (N=20) | |
| IL-6 (pg/mL) | 206.63 (66.50–387.54)* | 45.20 (22.95–112.55) | 51.98 (9.00–183.00) | 11.15 (1.00–93.12) |
| IL-10 (pg/mL) | 24.95 (10.13–66.40)‘ | 8.59 (5.02–22.26) | 12.58 (9.83–17.02)‘ | 7.78 (3.95–11.67) |
Table 3.
Diagnostic performance of various parameters for the diagnosis of overt DIC (N=81)
Table 4.
Comparison of plasma IL-6 and IL-10 levels between survivors and non-survivors
|
Overt DIC |
Non-overt DIC |
|||
|---|---|---|---|---|
| Survivors (N=18) | Non-survivors (N=10) | Survivors (N=38) | Non-survivors (N=10) | |
| IL-6 (pg/mL) | 64.40 (20.87–119.41)† | 319.46 (152.13–391.40) | 22.15 (4.75–67.25)* | 146.70 (57.50–288.05) |
| IL-10 (pg/mL) | 11.31 (9.01–23.72)† | 33.76 (14.50–70.91) | 8.06 (4.54–16.94) | 21.13 (5.58–33.72) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download