Abstract
Background
The hemopoietic stem cells increase in number during the regeneration after chemotherapy or bone marrow transplantation (BMT). Although the proportion of hemopoietic stem cells and their differentiation have been studied by immunophenotyping using the flow cytometry, no substantial research efforts have been directed toward the regenerating marrow. We attempted to discover the proportions of undifferentiated stem cells, committed stem cells, B cell precursors, and myeloid precursors in the regenerating bone marrows during complete remission (CR) and after engraftment of BMT.
Methods
Bone marrow samples from 82 patients with acute leukemia in CR and from 25 patients after BMT engraftment, along with 22 control samples, were used to find the numbers of CD38-/CD34+, CD38+/CD34+, CD19+/CD34+, and CD13,33+/CD34+ cells in the large lymphocyte gate by flow cytometry. We cross-analyzed our results in terms of groups: CR, BMT, and initial diagnosis groups. We performed significance tests on age, relapse, chromosomal abnormalities, clinical outcomes, and initial immunophenotypes of the leukemic cells.
Results
The proportions of CD38-/CD34+, CD38+/CD34+, CD19+/CD34+, and CD13,33+/CD34+ cells are more highly distributed in acute B-lymphoblastic leukemia than the normal group and also in the CR than the BMT group. CD19+/CD34+ cells were increased in the relapse group and CD38+/CD34+, CD19+/CD34+, and CD13,33+/CD34+ cells were increased in the group with chromosomal abnormality. The results were irrelevant to the initial immunophenotype of the leukemic blasts.
Go to : 
REFERENCES
1.LeBien TW., Wormann B., Villablanca JG., Law CL., Steinberg LM., Shah VO, et al. Multiparameter flow cytometric analysis of human fetal bone marrow B cells. Leukemia. 1990. 4:354–8.
2.Kern W., Danhauser-Riedl S., Ratei R., Schnittger S., Schoch C., Kolb HJ, et al. Detection of minimal residual disease in unselected patients with acute myeloid leukemia using multiparameter flow cytometry to definition of leukemia-associated immunophenotypes and determination of their frequencies in normal bone marrow. Haematologica. 2003. 88:646–53.
3.Ciudad J., Orfao A., Vidriales B., Macedo A., Martiinez A., Gonzalez M, et al. Immunophenotypic analysis of CD19+ precursors in normal human adult bone marrow: implications for minimal residual disease detection. Haematologica. 1998. 83:1069–75.
4.Macedo A., Orfao A., Ciudad J., Gonzalez M., Vidriales B., Lopez-Berges MC, et al. Phenotypic analysis of CD34 subpopulations in normal human bone marrow and its application for the detection of minimal residual disease. Leukemia. 1995. 9:1896–901.
5.Dworzak MN., Fritsch G., Fleischer C., Printz D., Fröschl G., Buchinger P, et al. Multiparameter phenotype mapping of normal and post-chemotherapy B lymphopoiesis in pediatric bone marrow. Leukemia. 1997. 11:1266–73.

6.van Wering ER., van der Linden-Schrever BE., Szczepanski T., Villemse MJ., Baars EA., van Wijngaarde-Schmitz HM, et al. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: implications for monitoring of minimal residual disease. Br J Haematol. 2000. 110:139–46.

7.Campana D., Pui CH. Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood. 1995. 85:1416–34.
8.Coustan-Smith E., Behm FG., Sanchez J., Boyett JM., Hancock ML., Raimondi SC, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998. 351:550–4.

9.Osawa M., Hanada K., Hamada H., Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996. 273:242–5.

10.Krause SD., Fackler MJ., Civin CI., May WS. CD34: structure, biology and clinical utility. Blood. 1996. 87:1–13.
11.Terstappen LW., Huang S., Safford D., Lansdorp PM., Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991. 77:1218–27.

12.McKenzie JL., Gan OI., Doedens M., Dick JE. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp Hematol. 2007. 35:1429–36.

13.van Rhenen A., Feller N., Kelder A., Westra AH., Rombouts E., Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005. 11:6520–7.

14.Monreal MB., Pardo MI., Pavlovsky MA., Fernandez I., Corrado CS., Giere I, et al. Increased immature hematopoietic progenitor cells CD34+/CD38dim in myelodysplasia. Cytometry B Clin Cytom. 2006. 70:63–70.
15.Smedmyr B., Bengtsson M., Jakobsson A., Simonsson B., Oberg G., Totterman TH. Regeneration of CALLA (CD10+), TdT+ and double-positive cells in the bone marrow and blood after autologous bone marrow transplantation. Eur J Haematol. 1991. 46:146–51.

16.Fukushima T., Sumazaki R., Koike K., Tsuchida M., Matsui A., Nakauchi H. Multicolor flow-cytometric, morphologic, and clonogenic analysis of marrow CD10-positive cells in children with leukemia in remission or nonmalignant diseases. J Pediatr Hematol Oncol. 1998. 20:222–8.

17.Andrews RG., Singer JW., Bernstein ID. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989. 169:1721–31.

18.Frixos Paraskevas'. Clusters of Differentiation. Greer JP, Foerster J, editors. Wintrobe's clinical hematology. 11th ed.Philadelphia: Lippincott;2004. p. 27–98.
Go to : 
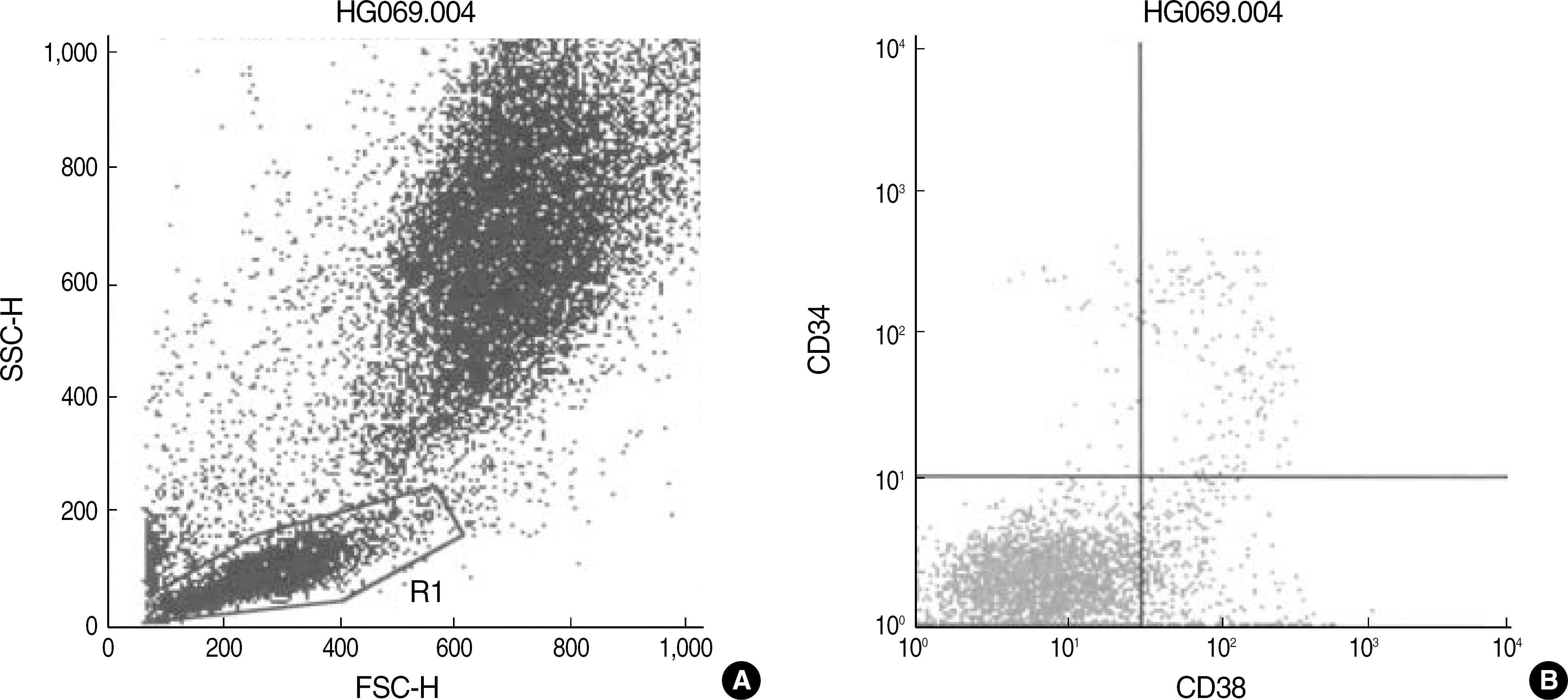 | Fig. 1.Dot plot scattergrams of bone marrow cells of a B-ALL patient in complete remission stained with anti-CD38-FITC/anti-CD34-PerCP (A, B). The large lymphocyte gate was set using forward scatter (FSC) and side scatter (SSC) characteristics (A). The CD38+/CD34+ cells in the gate (upper right quadrant) was 4.30% (B). |
Table 1.
Frequencies of cells expressing CD38-/CD34+, CD38+/CD34+, CD19+/CD34+, or CD13,33+/CD34+ in the bone marrow during complete remission of acute leukemia or after bone marrow transplantation
Table 2.
The differences of the frequencies of cells expressing CD38-/CD34+, CD38+/CD34+, CD19+/CD34+, or CD13,33+/CD34+ according to the variables




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download