Abstract
Background
Angiogenesis and osteoclastogenesis are increased in the bone marrow of multiple myeloma (MM) patients in parallel with the tumor progression. Osteopontin (OPN) is a multifunctional protein that is involved in angiogenesis and bone destruction and, eventually, in tumor progression in MM. OPN is known to increase in MM patients as the disease progresses and bone is destroyed. We studied the clinical usefulness of OPN as a monitoring marker for treatment response in patients with MM.
Methods
We obtained 70 serial sera from 27 MM patients and 14 sera from healthy individuals. OPN was measured by a sandwich ELISA method. The hospital records were reviewed, and the clinically important markers for monitoring the treatment response, such as monoclonal component, immunoglobulin, free light chain, and hemoglobin, etc, were analyzed together with OPN levels.
Results
There was no significant difference in OPN levels between MM patients and healthy controls. OPN showed no significant correlations with the markers used for monitoring of treatment response such as M component, immunoglobulin, and free light chain levels. There was no difference in OPN levels between the 3 groups classified by the amount of M component. In addition, OPN levels showed no compatible changes to the treatment response of MM patients.
Conclusions
Although OPN has been known to have an important role in the formation and progression of MM by involving angiogenesis and bone destruction, our results show that OPN is not valuable as a clinical marker for monitoring the treatment response in MM patients because of inconsistency in its levels in MM patients.
Go to : 
REFERENCES
1.De Raeve HR., Vanderkerken K. The role of the bone marrow microenvironment in multiple myeloma. Histol Histopathol. 2005. 20:1227–50.
2.Colla S., Morandi F., Lazzaretti M., Rizzato R., Lunghi P., Bonomini S, et al. Human myeloma cells express the bone regulating gene Runx2/Cbfa1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia. 2005. 19:2166–76.
3.Jakob C., Sterz J., Zavrski I., Heider U., Kleeberg L., Fleissner C, et al. Angiogenesis in multiple myeloma. Eur J Cancer. 2006. 42:1581–90.

4.Tanaka Y., Abe M., Hiasa M., Oda A., Amou H., Nakano A, et al. Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res. 2007. 13:816–23.

5.Mazzali M., Kipari T., Ophascharoensuk V., Wesson JA., Johnson R., Hughes J. Osteopontin–a molecular for all seasons. QJM. 2002. 95:3–13.
6.Cheriyath V., Hussein MA. Osteopontin, angiogenesis and multiple myeloma. Leukemia. 2005. 19:2203–5.

7.Weidner N., Semple JP., Welch WR., Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.
8.Salven P., Teerenhovi L., Joensuu H. A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma. Blood. 1997. 90:3167–72.

9.Borset M., Lien E., Espevik T., Helseth E., Waage A., Sundan A. Concomitant expression of hepatocyte growth factor/scatter factor and the receptor c-MET in human myeloma cell lines. J Biol Chem. 1996. 271:24655–61.
10.Seidel C., Borset M., Hjorth-Hansen H., Sundan A., Waage A. Role of hepatocyte growth factor and its receptor c-met in multiple myeloma. Med Oncol. 1998. 15:145–53.
11.Sato N., Hattori Y., Wenlin D., Yamada T., Kamata T., Kakimoto T, et al. Elevated level of plasma basic fibroblast growth factor in multiple myeloma correlates with increased disease activity. Jpn J Cancer Res. 2002. 93:459–66.

12.Iwasaki T., Hamano T., Ogata A., Hashimoto N., Kitano M., Kakishita E. Clinical significance of vascular endothelial growth factor and hepatocyte growth factor in multiple myeloma. Br J Haematol. 2002. 116:796–802.

13.Di Raimondo F., Azzaro MP., Palumbo G., Bagnato S., Giustolisi G., Floridia P, et al. Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica. 2000. 85:800–5.
14.Saeki Y., Mima T., Ishii T., Ogata A., Kobayashi H., Ohshima S, et al. Enhanced production of osteopontin in multiple myeloma: clinical and pathogenic implications. Br J Haematol. 2003. 123:263–70.
15.Durie BG., Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975. 36:842–54.

16.Blade J., Samson D., Reece D., Apperley ., Bjorkstrand B., Gahrton G, et al. Criteria for evaluating disease response and progression in patient with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998. 102:1115–23.
17.Haylock DN., Nilsson SK. Osteopontin: a bridge between bone and blood. Br J Haematol. 2006. 134:467–74.

18.Vacca A., Ribatti D., Roncali L., Ranieri G., Serio G., Silvestris F, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994. 87:503–8.

19.Sezer O., Jakob C., Eucker J., Niemoller K., Gatz F., Wernecke K, et al. Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur J Haematol. 2001. 66:83–8.

20.Standal T., Hjorth-Hansen H., Rasmussen T., Dahl IM., Lenhoff S., Brenne AT, et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica. 2004. 89:174–82.
21.Kang SY., Suh JT., Lee HJ., Yoon HJ., Lee WI. Establishment of serum reference range for free light chains and its clinical usefulness in multiple myeloma. Korean J Lab Med. 2004. 24:273–8. (강소영, 서진태, 이희주, 윤휘중, 이우인. 혈청유리형경쇄참고치설정과다발성골수종환자들에서의임상적의의. 대한진단검사의학회지 2004;24: 273-8.).
Go to : 
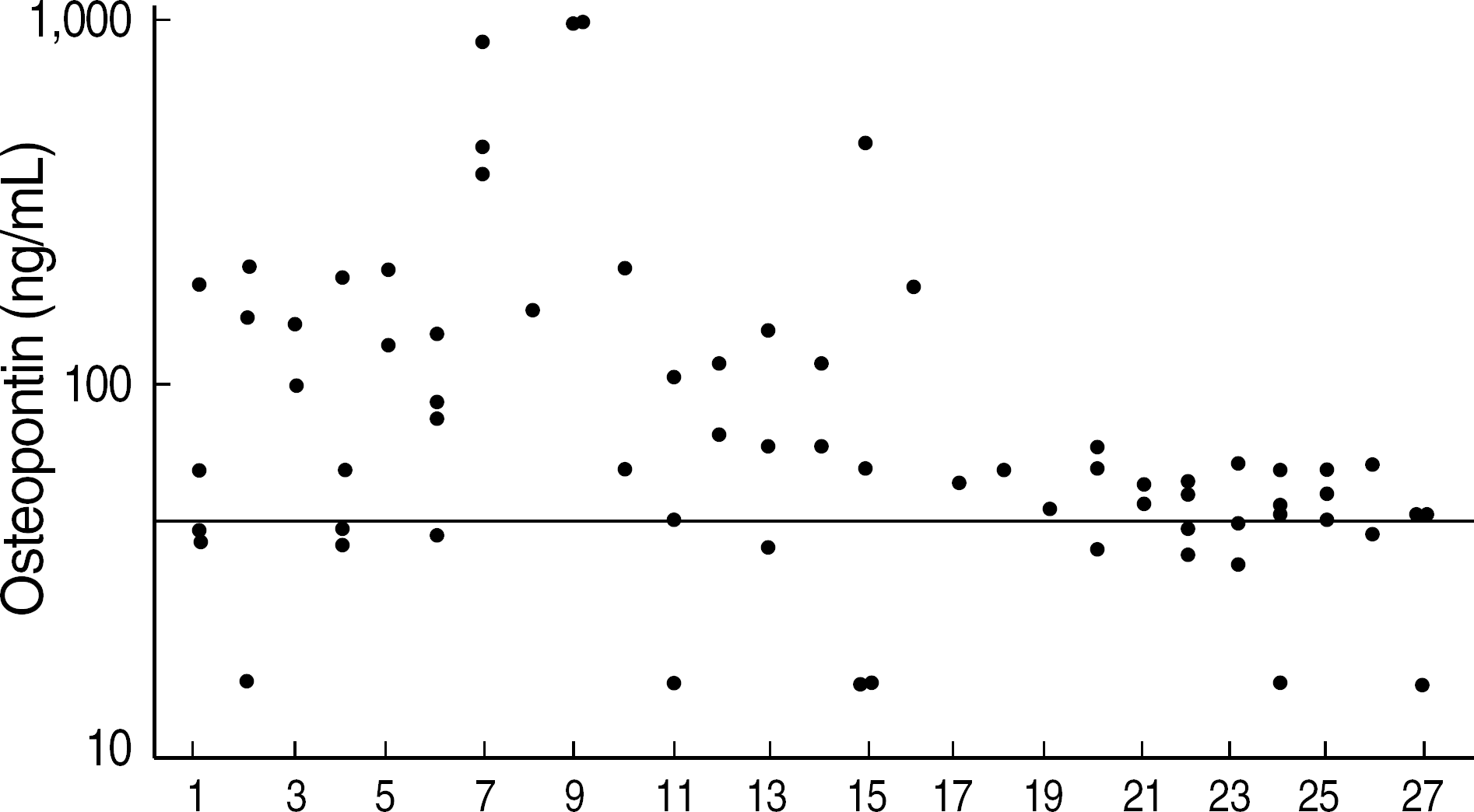 | Fig. 1.The distributions of osteopontin concentrations in serial sera of 27 multiple myeloma patients.
Line, cut-off value (41.8 ng/mL) by ROC curve analysis.
|
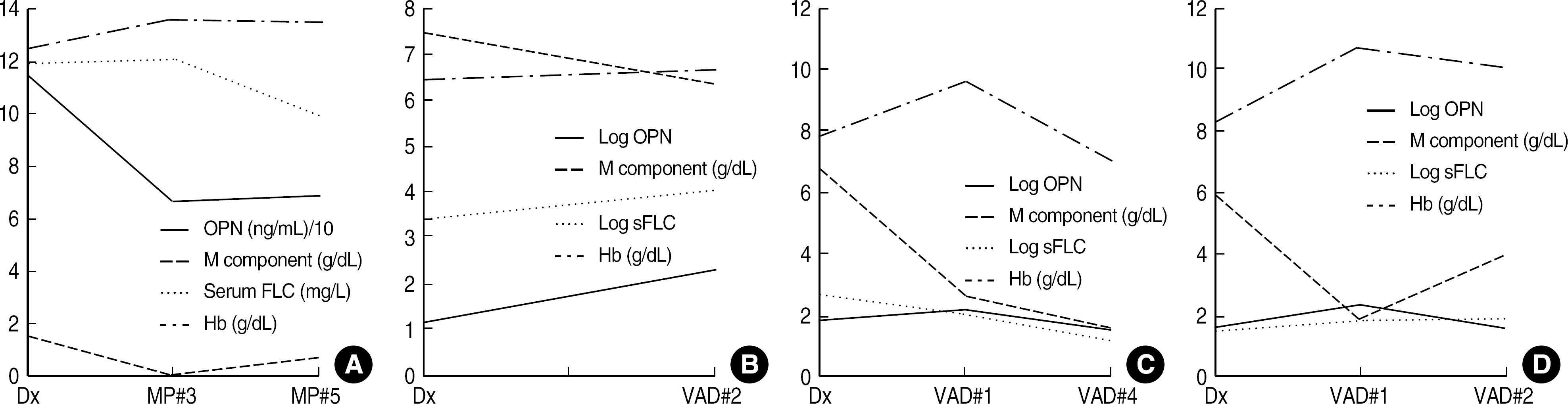 | Fig. 2.The distributions of clinical markers used for monitoring the treatment response in each multiple myeloma patient. (A) Complete response, (B) No change, (C) and (D) Partial response. Abbreviations: MP, melphalan, prednisolone; VAD, vincristine, adriamycin, dexamethasone; OPN, osteopontin; See Table 1. |
Table 1.
The correlation between osteopontin and clinical markers
Table 2.
The comparison of clinical markers used for monitoring the treatment response between 3 groups classified by amount of M component




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download