Abstract
Background
To enumerate leukocyte count in cerebrospinal fluid (CSF) is important for diagnosing bacterial meningitis. Using automated hematology analyzer for enumeration of leukocyte in CSF is below the sensitivity, so microscopic hemocytometric method is standard method. But this requires sufficient practical experience and has limitation of accuracy and stability. So we developed new manual method and evaluated it.
Methods
We designed new method using transparent ruler tape. We performed correlation, accuracy and precision test by counting leukocyte in diluted EDTA blood with three methods: new method, Neubauer and Nageotte hemocytometry. Twenty two CSF were used for stability test, which determines leukocyte count according to time (within one hour and after 2, 4 and 12 hr), by new method and Neubauer hemocytometry at room temperature.
Results
There was no clinical significant difference between three methods in correlation test, whereas Neubauer and Nageotte hemocytometry showed a bias to underestimation relative to the results obtained with new method in case with low leukocyte count. The new method showed the lowest CV and most accurate result. In stability test, leukocyte counts decreased being 44.4%, 72.1% of initial values after 2 hr, 14.8%, 31.1%, after 4 hr and 4.2%, 8.7%, after 12 hr, by Nageotte hemocytometry and new method, respectively.
Go to : 
REFERENCES
1.Hoen B., Viel JF., Paquot C., Gerard A., Canton P. Multivariate approach to differential diagnosis of acute meningitis. Eur J Clin Microbiol Infect Dis. 1995. 14:267–74.

2.Muller TH., Doscher A., Schunter F., Scott CS. Manual and automated methods for the determination of leukocyte counts at extreme low levels: comparative evaluation of the Nageotte chamber and the Abbott Cell Dyn 3500 analyser. Transfus Sci. 1997. 18:505–15.
3.Chow G., Schmidley JW. Lysis of erythrocytes and leukocytes in traumatic lumbar punctures. Arch Neurol. 1984. 41:1084–5.

4.Veerman AJ., Huismans L., van Zantwijk I. Storage of cerebrospinal fluid samples at room temperature. Acta Cytol. 1985. 29:188–9.
5.Steele RW., Marmer DJ., O'Brien MD., Tyson ST., Steele CR. Leukocyte survival in cerebrospinal fluid. J Clin Microbiol. 1986. 23:965–6.

6.Ziebig R., Lun A., Sinha P. Leukocyte counts in cerebrospinal fluid with the automated hematology analyzer CellDyn 3500 and the urine flow cytometer UF-100. Clin Chem. 2000. 46:242–7.

7.Andrews J., Setran E., McDonnel L., Kussick S., Wood BL., Sabath DE. An evaluation of the cell-dyn 3200 for counting cells in cerebrospinal and other body fluids. Lab Hematol. 2005. 11:98–106.

8.Van Acker JT., Delanghe JR., Langlois MR., Taes YE., De Buyzere ML., Verstraete AG. Automated flow cytometric analysis of cerebrospinal fluid. Clin Chem. 2001. 47:556–60.

9.Brecher ME., Harbaugh CA., Pineda AA. Accurate counting of low numbers of leukocytes. Use of flow cytometry and a manual low-count chamber. Am J Clin Pathol. 1992. 97:872–5.

10.Dzik S., Moroff G., Dumont L. A multicenter study evaluating three methods for counting residual WBCs in WBC-reduced blood components: Nageotte hemocytometry, flow cytometry, and microfluorometry. Transfusion. 2000. 40:513–20.

11.Borzini P., Dumont LJ. Microdroplet fluorochromatic assay for the enumeration of white cells (WBCs) in WBC-reduced blood components: validation and application for evaluating newly developed WBC-reduction filters. Transfusion. 1997. 37:601–6.

12.Seghatchian J., Krailadsiri P., Scott CS. Counting of residual WBCs in WBC-reduced blood components: a multicenter evaluation of a microvolume fluorimeter by the United Kingdom National Blood Service. Transfusion. 2001. 41:93–101.

13.Cassens U., Greve B., Tapernon K., Nave B., Severin E., Sibrowski W, et al. A novel true volumetric method for the determination of residual leucocytes in blood components. Vox Sang. 2002. 82:198–206.

14.Watson MA., Scott MG. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin Chem. 1995. 41:343–60.

15.Spanos A., Harrell FE Jr., Durack DT. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA. 1989. 262:2700–7.

17.Fishbein DB., Palmer DL., Porter KM., Reed WP. Bacterial meningitis in the absence of CSF pleocytosis. Arch Intern Med. 1981. 141:1369–72.

18.Lindquist L., Linne T., Hansson LO., Kalin M., Axelsson G. Value of cerebrospinal fluid analysis in the differential diagnosis of meningitis: a study in 710 patients with suspected central nervous system infection. Eur J Clin Microbiol Infect Dis. 1988. 7:374–80.

19.Nye FJ. The value of initial laboratory investigations in the management of meningitis. J Infect. 1983. 7:31–8.

20.Aulesa C., Mainar I., Prieto M., Cobos N., Galimany R. Use of the Advia 120 hematology analyzer in the differential cytologic analysis of biological fluids (cerebrospinal, peritoneal, pleural, pericardial, synovial, and others). Lab Hematol. 2003. 9:214–24.
Go to : 
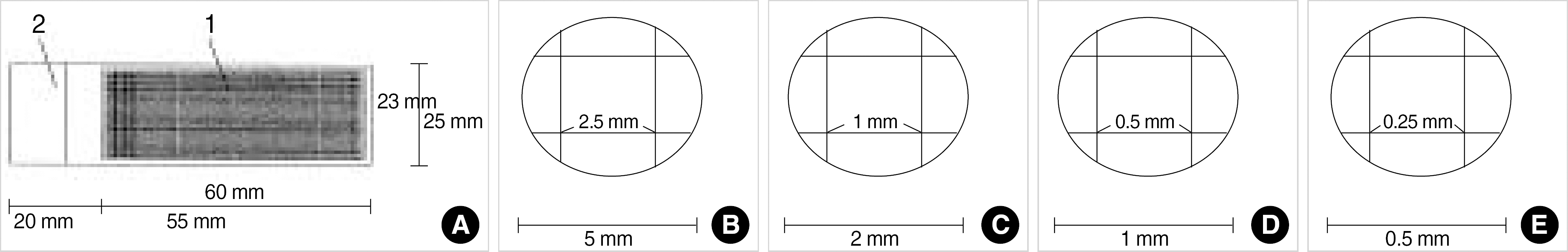
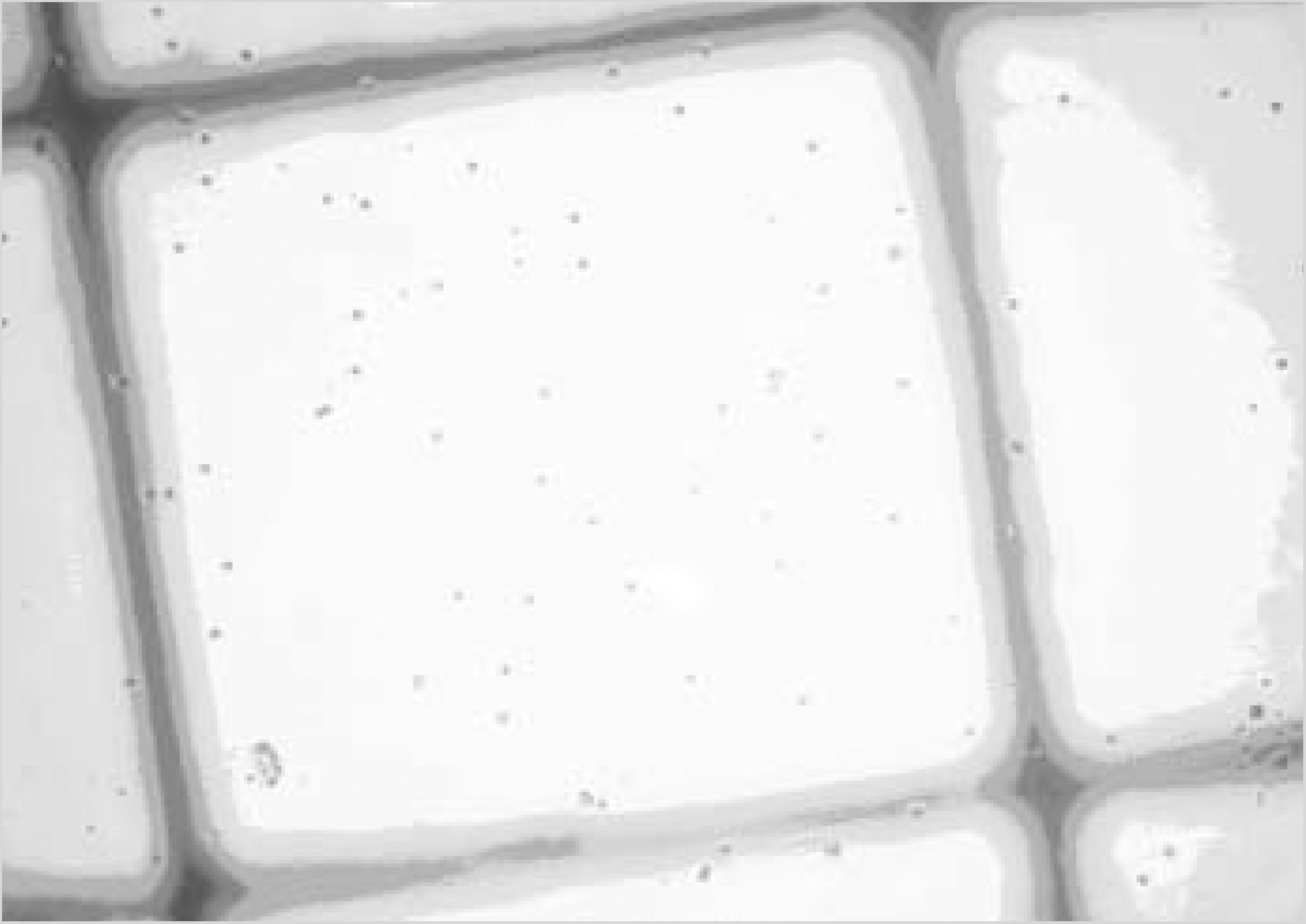
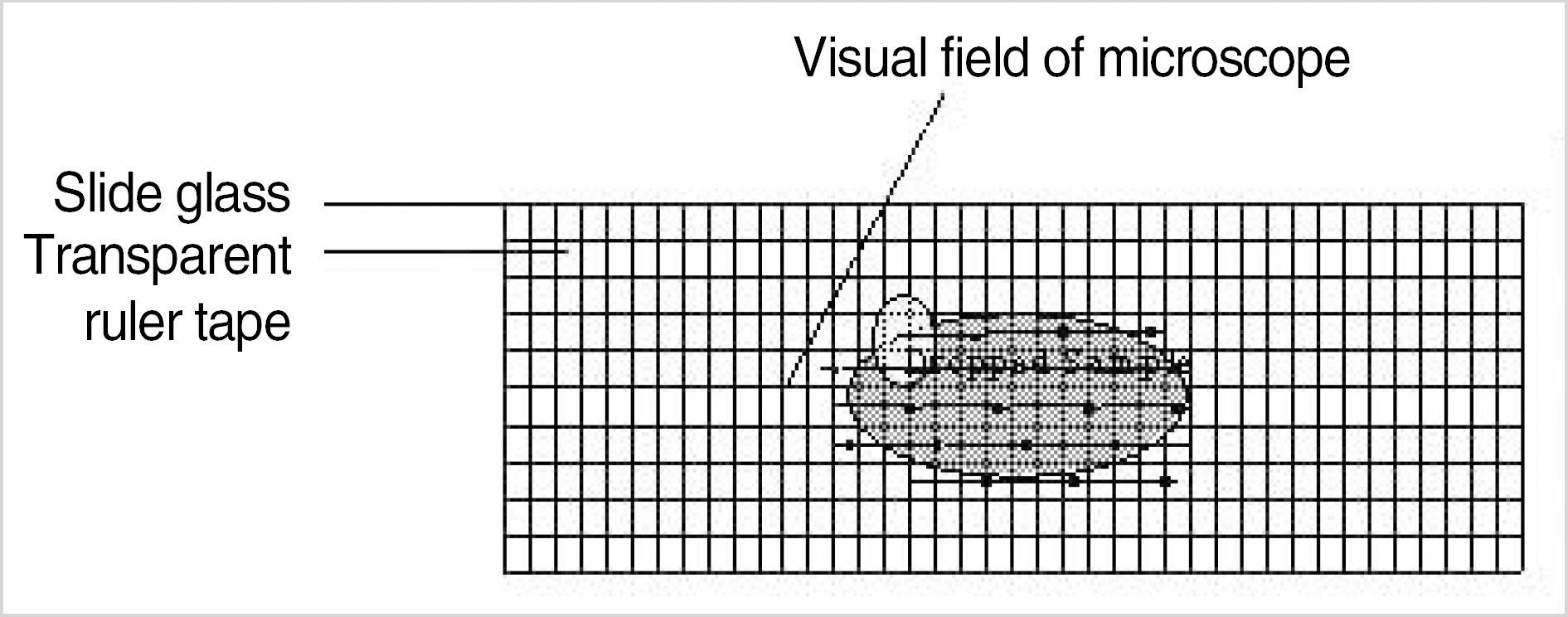 | Fig. 1.Slide with transparent ruler tape for cell count (A), visual fields of microscope ×40 (B), ×100 (C), ×200 (D), ×400 (E). |
 | Fig. 2.Schematic figure of new counting method. The arrow indicates direction in microscopic examination. |
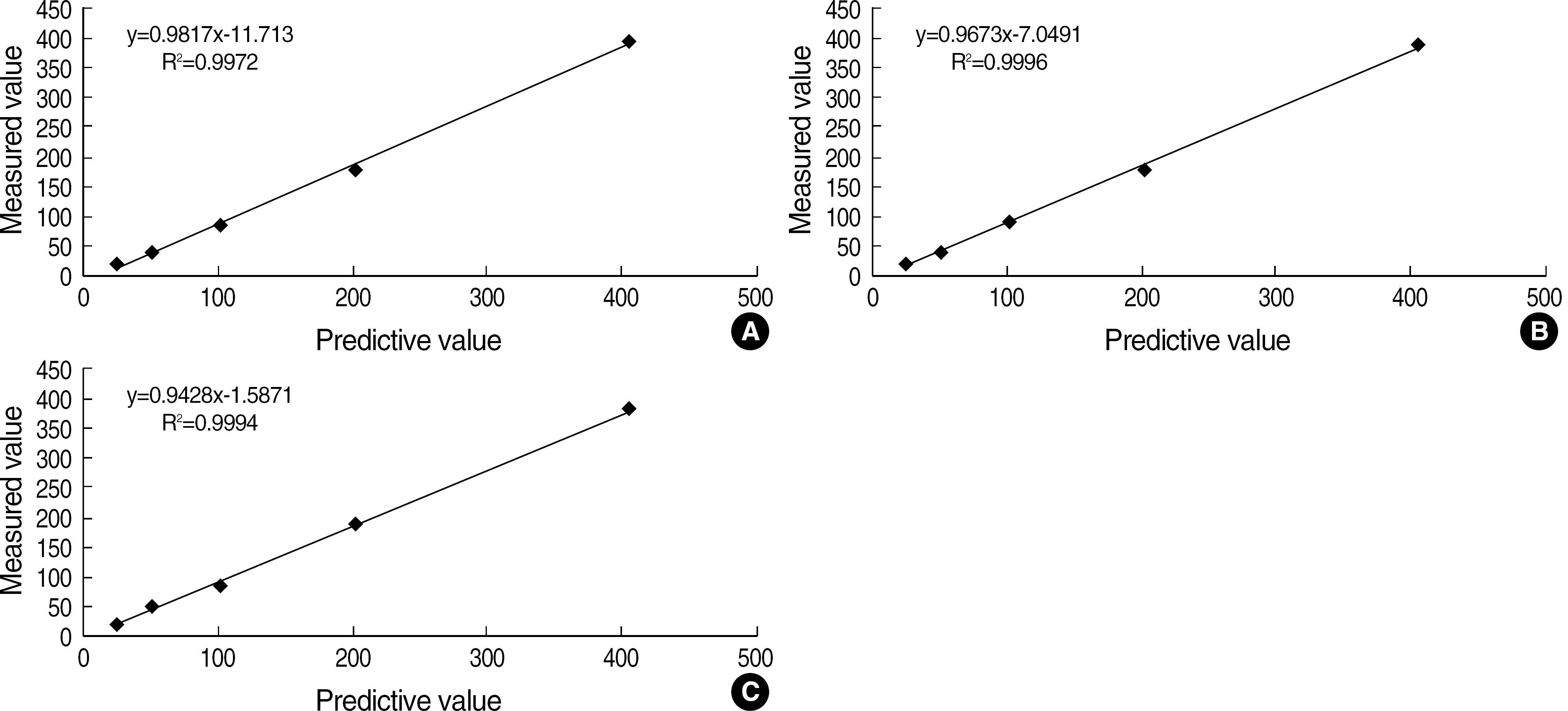 | Fig. 4.Linearity of leukocyte counting with three method; Neubauer hemacytometer (A), Nageotte chamber (B), new counting method (C) using diluents of EDTA blood samples. Each point represent an individual observation (N=10). |
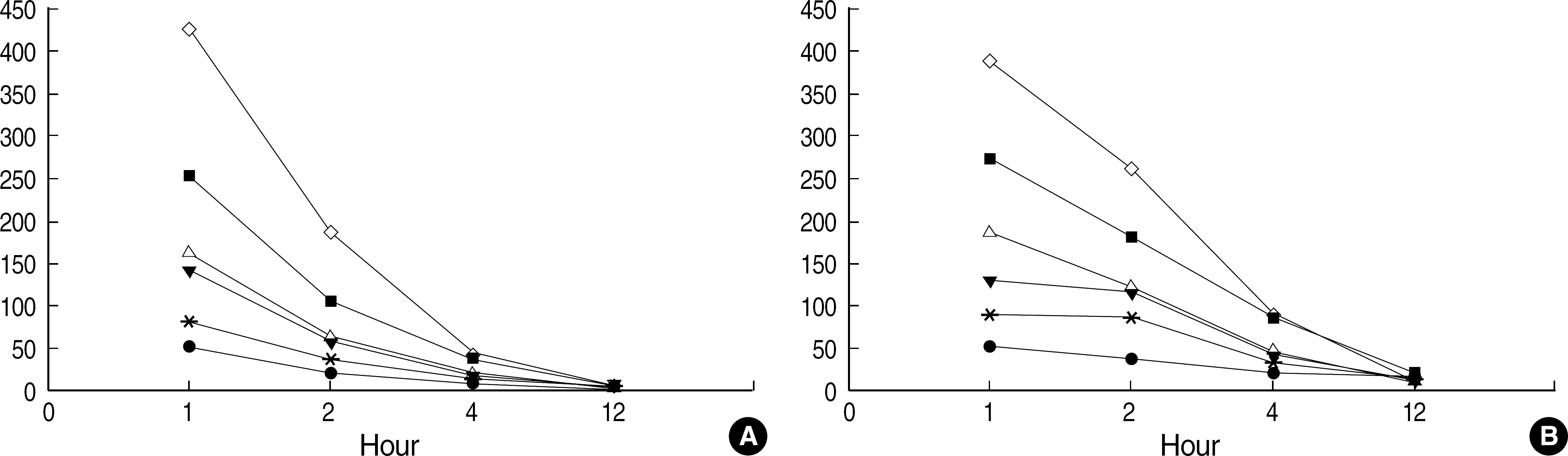 | Fig. 5.Leukocyte count (/μL) change with Nageotte chamber (A) and new counting method (B) of each samples (6 cases of initial WBC count >50/μL) according to time elapse. |
Table 1.
Comparison of methods for white blood cell enumeration applied on Neubauer hemocytometer, Nageotte chamber counting and New method by using a different amounts of diluents of EDTA blood
Table 2.
Comparison of methods for white blood cell enumeration by Nageotte chamber and New method during storage at room temperature




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download