Abstract
Background
Following induction chemotherapy for AML, a sensitive determination of minimal residual disease (MRD) in patients achieving complete remission (CR) should enable the detection of early relapse. This study was designed to verify if quantitative assessment of the Wilms’ tumor (WT1) gene by real time polymerase chain reaction (RQ-PCR) can be used as a marker for MRD detection during the monitoring of AML.
Methods
WT1 gene expression was quantified by RQ-PCR in 31 patients with AML at diagnosis (27 patients) and during follow-up (29 patients) relative to ABL control gene. In four patients, the WT1 gene expression was analyzed in comparison to a second PCR marker, PML-RARA fusion transcript. Prognostic significance of WT1 gene expression was analyzed at diagnosis and at the primary CR evaluation. Longitudinal WT1 gene analysis was performed in 17 AML patients.
Results
At diagnosis, WT1 gene expression exceeded the control level in all of the patients. Higher levels of WT1 gene expression were not associated with shorter event free survival or overall survival at diagnosis. Higher levels of WT1 gene expression were associated with shorter event free survival after induction chemotherapy. Relapse was observed in eight of 17 patients analysed longitudinally, and an increase of WT1 gene expression preceded morphologic relapse in four patients with the fusion transcript negative. Concomitant monitoring of PML-RARA fusion transcript reveals the lack of a significant correlation with WT1 gene expression.
Go to : 
REFERENCES
1.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002. 29:23–39.

2.van der Velden VH., Hochhaus A., Cazzaniga G., Szczepanski T., Gabert J., van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003. 17:1013–34.

3.Ostergaard M., Olesen LH., Hasle H., Kjeldsen E., Hokland P. WT1 gene expression: an excellent tool for monitoring minimal residual disease in 70% of acute myeloid leukaemia patients-results from a single-centre study. Br J Haematol. 2004. 125:590–600.
4.Lin F., van Rhee F., Goldman JM., Cross NC. Kinetics of increasing BCR-ABL transcript numbers in chronic myeloid leukemia patients who relapse after bone marrow transplantation. Blood. 1996. 87:4473–8.
5.Olavarria E., Kanfer E., Szydlo R., Kaeda J., Rezvani K., Cwynarski K, et al. Early detection of BCR-ABL transcripts by quantitative reverse transcriptase-polymerase chain reaction predicts outcome after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2001. 97:1560–5.
6.Ogawa H., Tamaki H., Ikegame K., Soma T., Kawakami M., Tsuboi A, et al. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood. 2003. 101:1698–704.
7.Pallisgaard N., Hokland P., Riishoj DC., Pedersen B., Jorgensen P. Multiplex reverse transcription-polymerase chain reaction for simultaneous screening of 29 translocations and chromosomal aberrations in acute leukemia. Blood. 1998. 92:574–88.

8.Inoue K., Sugiyama H., Ogawa H., Nakagawa M., Yamagami T., Miwa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994. 84:3071–9.
9.Inoue K., Ogawa H., Sonoda Y., Kimura T., Sakabe H., Oka Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997. 89:1405–12.
10.Miwa H., Beran M., Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992. 6:405–9.
11.Miyagi T., Ahuja H., Kudota T. Expression of the candidate Wilms' tumor gene, WT1, in human leukemia cells. Leukemia. 1993. 7:970–7.
12.Brieger J., Weidmann E., Fenchel K., Mitrou PS., Hoelzer D., Bergmann L. The expression of the Wilms' tumor gene in acute myelocytic leukemias as a possible marker for leukemic blast cells. Leukemia. 1994. 8:2138–43.
13.Menssen HD., Renkl HJ., Rodeck U., Maurer J., Notter M., Schwartz S, et al. Presence of Wilms' tumor gene (WT1) transcripts and the WT1 nuclear protein in the majority of human acute leukemias. Leukemia. 1995. 9:1060–7.
14.Gessler M., Poustka A., Cavenee W., Neve RL., Orkin SH., Bruns GA. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990. 343:774–8.

15.Maurer U., Brieger J., Weidmann E., Mitrou PS., Hoelzer D., Bergmann L. The Wilms' tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro. Exp Hematol. 1997. 25:945–50.
16.Algar EM., Khromykh T., Smith SI., Blackburn DM., Bryson GJ., Smith PJ. A WT1 antisense oligonucleotide inhibits proliferation and induces apoptosis in myeloid leukaemia cell lines. Oncogene. 1996. 12:1005–14.
17.Svedberg H., Chylicki K., Baldetorp B., Rauscher FJ 3rd., Gullberg U. Constitutive expression of the Wilms' tumor gene (WT1) in the leukemic cell line U937 blocks parts of the differentiation program. Oncogene. 1998. 16:925–32.
18.Schmid D., Heinze G., Linnerth B., Tisljar K., Kusec R., Geissler K, et al. Prognostic significance of WT1 gene expression at diagnosis in adult de novo acute myeloid leukemia. Leukemia. 1997. 11:639–43.
19.Garg M., Moore H., Tobal K., Liu Yin JA. Prognostic significance of quantitative analysis of WT 1 gene transcripts by competitive reverse transcription polymerase chain reaction in acute leukaemia. Br J Haematol. 2003. 123:49–59.
20.Gaiger A., Linnerth B., Mann G., Schmid D., Heinze G., Tisljar K, et al. Wilms' tumour gene (WT1) expression at diagnosis has no prognostic relevance in childhood acute lymphoblastic leukaemia treated by an intensive chemotherapy protocol. Eur J Haematol. 1999. 63:86–93.
21.Bergmann L., Miething C., Maurer U., Brieger J., Karakas T., Weidmann E, et al. High levels of Wilms' tumor gene (WT1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997. 90:1217–25.
22.Grimwade D., Walker H., Oliver F., Wheatley K., Harrison C., Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998. 92:2322–33.
23.Gabert J., Beillard E., van der Velden VH., Bi W., Grimwade D., Pallisgaard N, et al. Standardization and quality control studies of ‘realtime’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – a Europe Against Cancer Program. Leukemia. 2003. 17:2318–57.

24.Weissor M., Kern W., Rauhut S., Schoch C., Hiddemann W., Haferlach T, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia. 2005. 19:1416–23.
25.Cassinat B., Zassadowski F., Balitrand N., Barbay C., Rain JD., Fenaux P, et al. Quantitation of minimal residual disease in acute promyelocytic leukemia patients with t(15;17) translocation using real-time RT-PCR. Leukemia. 2000. 14:324–8.

Go to : 
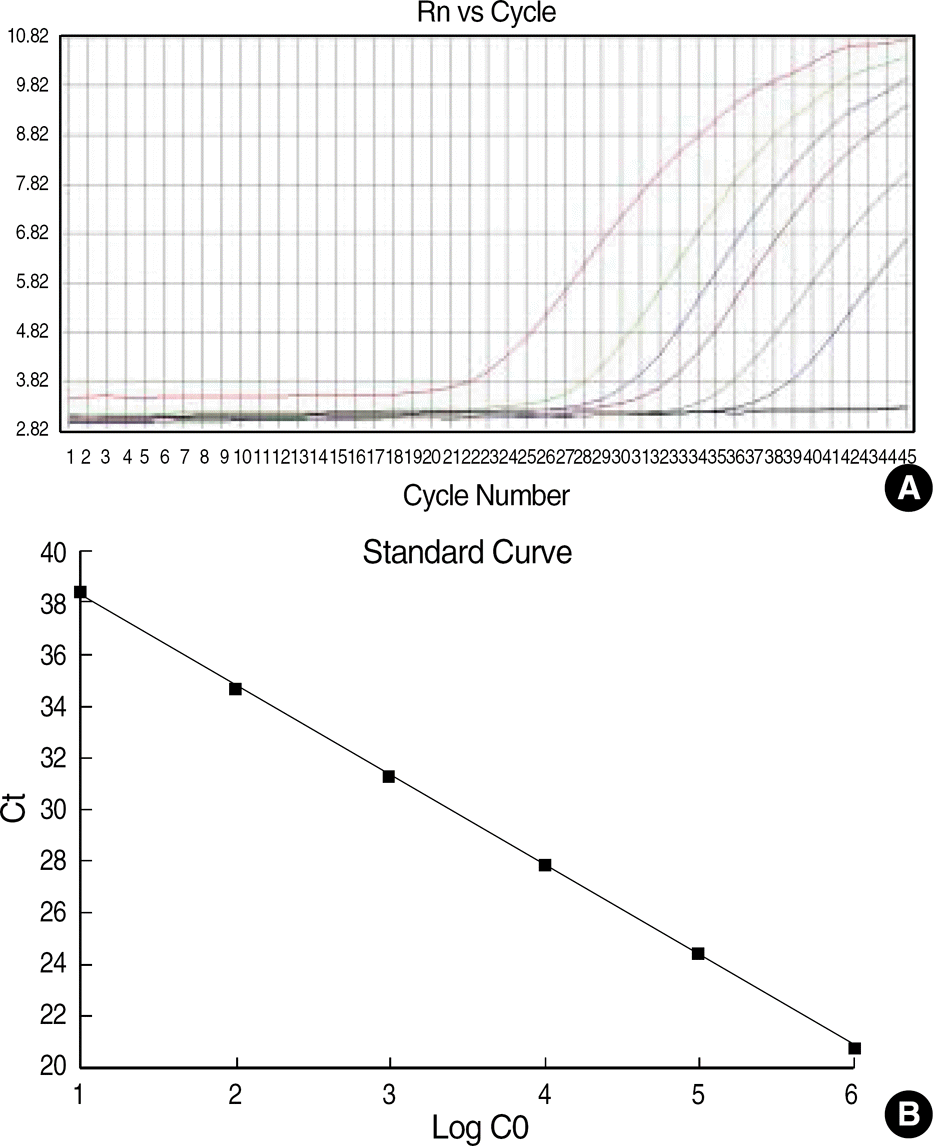 | Fig. 1.
WT1 amplication plot and standard curve. (A) Amplication plot of a 10-fold serial dilution of standard K562 cDNA (ranging from 10−5 to 100). The amplication plot shows an increase of reporter fluorescence during amplication. (x-axis, number of cycle; y-axis, relative fluorescence intensity). (B) Standard curve of K562 cDNA dilution for real-time PCR. The standard curve shows a linear correlation between the Ct value (y-axis) and the logarithm of the initial concentration of the standard K562 cDNA (x-axis). |
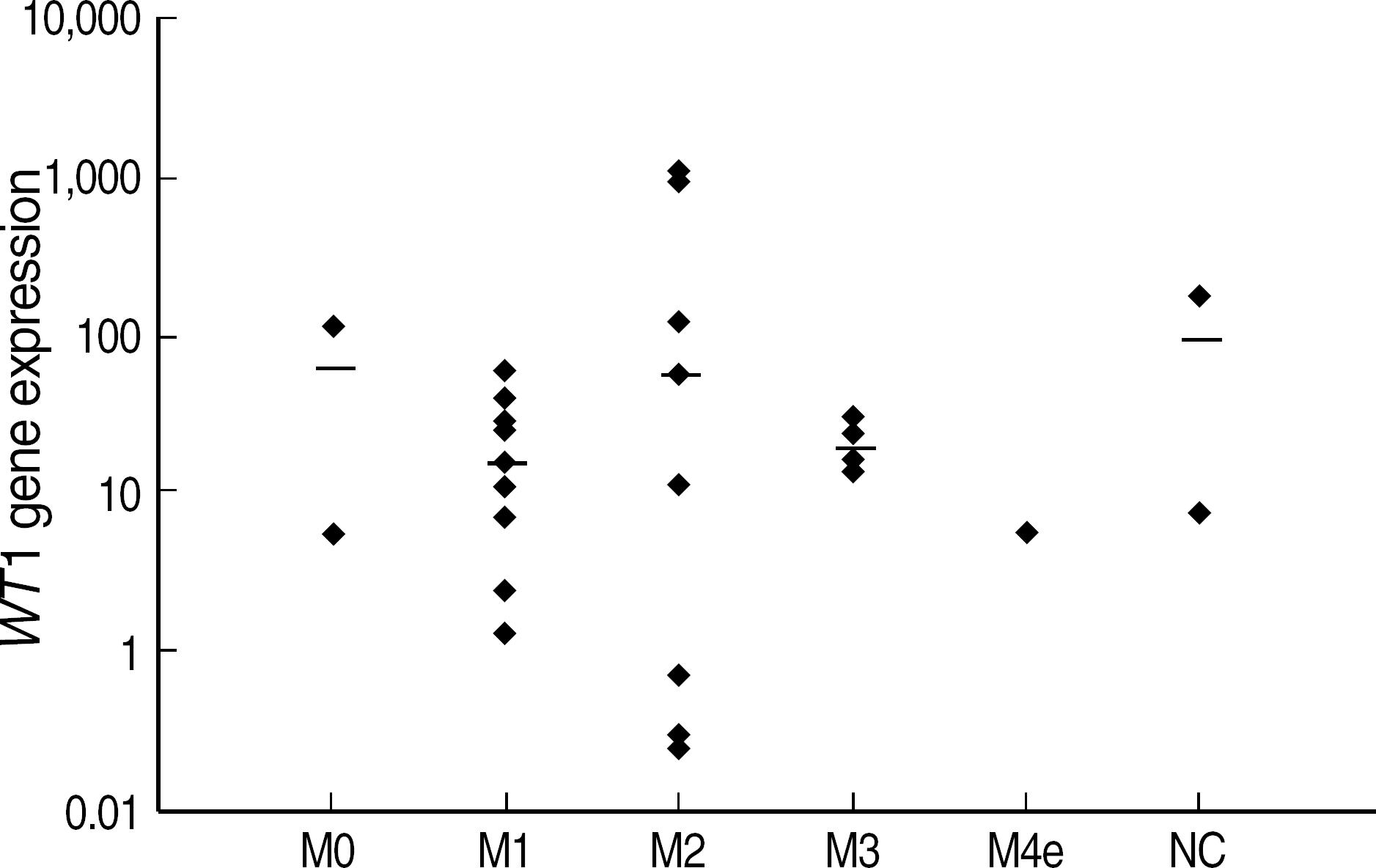 | Fig. 2.
WT1 expression at diagnosis (N=27) according to FAB subtypes. Abbreviations: NC, Not classifiable by FAB classification. |
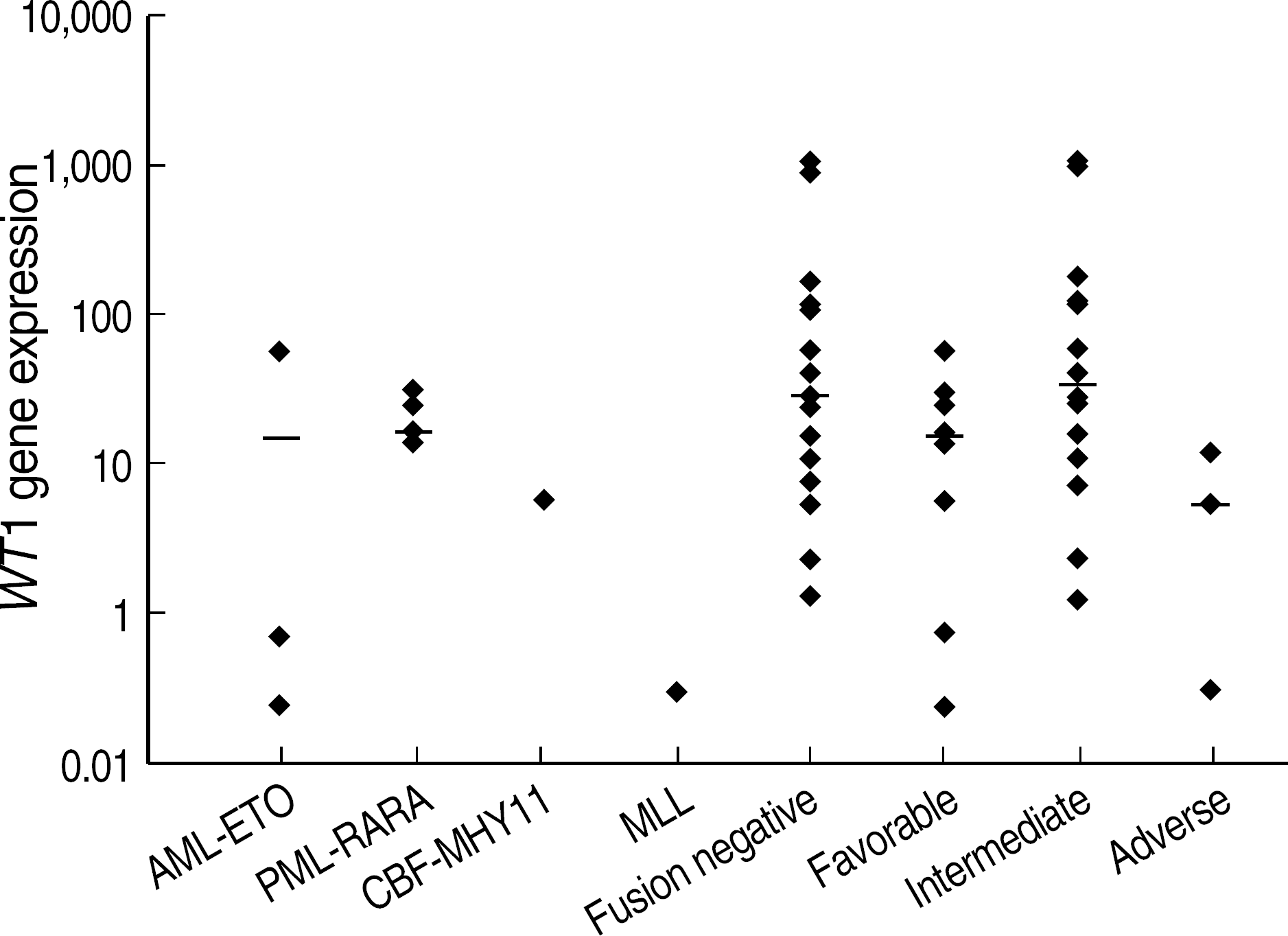 | Fig. 3.
WT1 expression at diagnosis (n=27) according to different molecular or cytogenetic prognostic subgroups of AML. Favorable: t(15;17), t(8;21), t(16;16); Intermediate: +8, +11, t(12;18) and normal karyotype; Adverse: −7, del(11)(q23), and complex karyotype as the presence of 3 or more abnormalities. Abbreviations: NC, Not classifiable by FAB classification. |
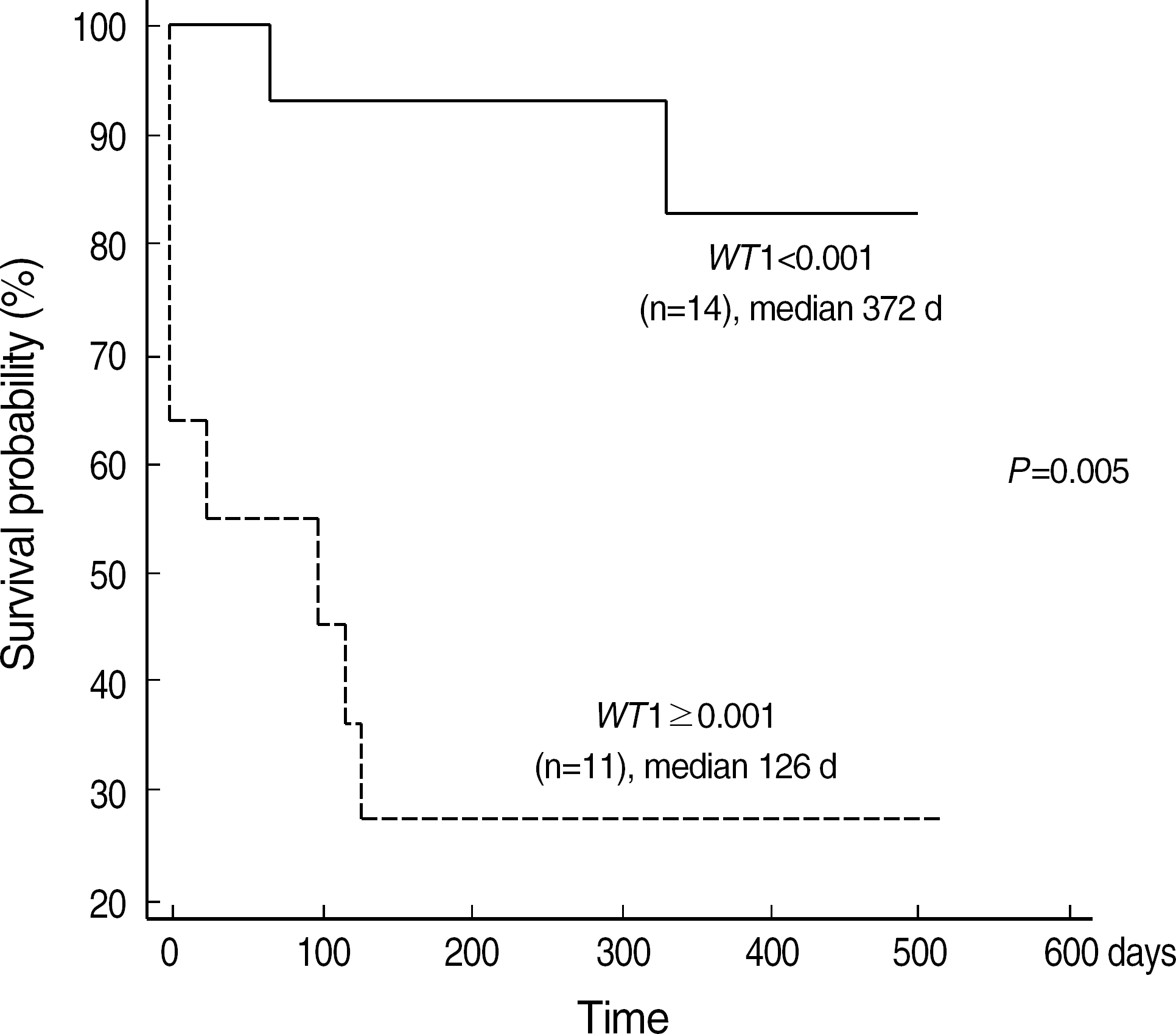 | Fig. 4.Kaplan-Meier plot of event free survival of patients with WT1 expression above or below 0.001. Abbreviations: NC, Not classifiable by FAB classification. |
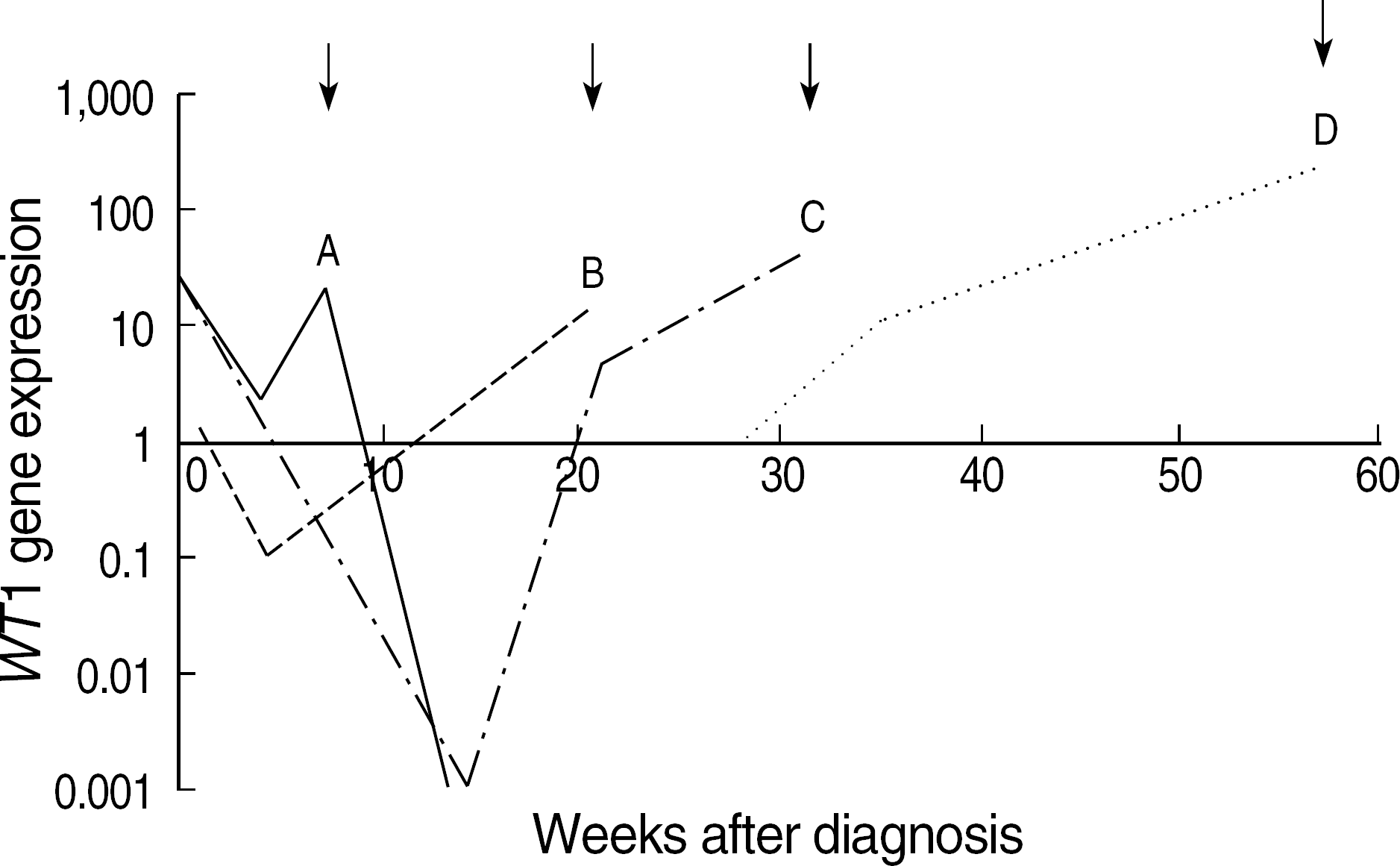 | Fig. 5.Longitudinal monitoring of WT1 level in four exemplary patients with relapse. Arrows on the top of the graph indicate the time points of hematologic relapse. Abbreviations: NC, Not classifiable by FAB classification. |
Table 1.
Clinical characteristics in patients with acute myelogenous leukemia
Table 2.
Primers and probes
| Primers and probes∗ | Sequence; 5′ – 3′ |
|---|---|
| WT1 forward primer | GAT AAC CAC ACA ACG CCC ATC |
| WT1 reverse primer | CAC ACG TCG CAC ATC CTG AAT |
| WT1 probe | ACA CCG TGC GTG TGT ATT CTG TAT TGG |
| ABL forward primer | TGG AGA TAA CAC TCT AAG CAT AAC TAA AGG |
| ABL reverse primer | GAT GTA GTT GCT TGG GAC CCA |
| ABL probe | CCA TTT TTG GTT TGG GCT TCA CAC CAT T |
| bcr 3 forward primer | AGC TCT TGC ATC ACC CAG GGG A |
| bcr 1 forward primer | GTC TTC CTG CCC AAC AGC AAC C |
| common reverse primer | CTC ACA GGC GCT GAC CCC ATA GT |
| PML-RARA probe | CAG CCC TCC CTC GCC ACC CCC TCT A |
Table 3.
Prognostic significance of WT1 expression above or below 0.001 following induction chemotherapy (N=25)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download