Abstract
Background
The multidrug resistance (mdr1), multidrug resistance associated protein (mrp1), and glutathione-s-transferase (gst) π genes have been associated with treatment failure in acute myeloid leukemia (AML). c-jun N-terminal kinase (JNK) activity is increased in response to chemotherapeutic agent.
Methods
To investigate the significance of multidrug resistance (mdr) parameters and JNK activity, bone marrow or peripheral blood cells from 52 patients with AML were analyzed. RT-PCR was performed for mdr1, mrp1, and gst π gene expression. JNK expression and activity were measured using an immunoenzymatic kinase assay and a western blot method.
Results
High level expression of mdr1, mrp1, and gst π mRNA was observed in 38.5%, 48.1% and 54.3% of AML cases, respectively. The remission rate was significantly low in cases with an older age (>55 yr), a high WBC count, poor chromosomal abnormalities, a high level expression of mdr1 and mrp1. The WBC count and mdr1 mRNA expression were independent predictors for the outcome to induction chemotherapy. There was a shorter duration of overall survival in the patients with an older age, a high WBC count, chromosome aberrations, high level expressions of mdr1 and mrp1 mRNA, and JNK activation. The patient's age, WBC count and chromosomal abnormalities were independent predictors for overall survivals. The majority (28/30) of AML cases did not show any levels of JNK activation except for two cases, which were associated with an extremely high WBC count, chromosomal aberration, high level expressions of mdr1, mrp1 and gst π mRNA, and treatment resistance.
Conclusions
These data indicate the influences of mdr1 and mrp1 mRNA expression on the clinical outcome of AML to induction chemotherapy. But it will be necessary to investigate further whether blast cells of AML resistant to chemotherapy retain the capacity to activate JNK, and relate to MDR parameters.
References
1. Zittoun R, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogenic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EO-RTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'-Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995; 332:217–23.
2. Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992; 258:1650–4.

3. Del Poeta G, Stasi R, Aronica G, Venditti A, Cox MC, Bruno A, et al. Clinical relevance of P-glycoprotein expression in de novo acute myeloid leukemia. Blood. 1996; 87:1997–2004.

4. Campos L, Guyotat D, Archimbaud E, Calmard-Oriol P, Tsuruo T, Troncy J, et al. Clinical significance of multidrug resistance P-glycoprotein expression on acute nonlymphoblastic leukemia cells at diagnosis. Blood. 1992; 79:473–6.
5. Sato H, Preisler H, Day R, Raza A, Larson R, Browman G, et al. MDR1 transcript levels as an indication of resistant disease in acute myelogenous leukaemia. Br J Haematol. 1990; 75:340–5.
6. Baek JH, Park SW, Kim DH, Jung JT, Kwak DS, Park SH, et al. The frequency and clinical significance of multidrug resistance-1(MDR-1) gene expression in acute myeloid leukemia. Korean J Hematol. 2000; 35:117–25.
7. Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T, SHG AML 96 study Group. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol. 2005; 128:324–32.
8. Zhou DC, Zittoun R, Marie JP. Expression of multidrug resistance-associated protein (MRP) and multidrug resistance (MDR1) genes in acute myeloid leukemia. Leukemia. 1995; 9:1661–6.
9. Hunault M, Zhou D, Delmer A, Ramond S, Viguie F, Cadiou M, et al. Multidrug resistance gene expression in acute myeloid leukemia: major prognossis significance for in vivo drug resistance to induction treatment. Ann Hematol. 1997; 74:65–71.
10. Zaman GJ, Lankelma J, van Tellingen O, Beijnen J, Dekker H, Paulusma C, et al. Role of glutathione in the export of compounds form cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci USA. 1995; 92:7690–4.
11. Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, et al. Expression of multidrug resistance protein/GS-X pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem. 1998; 273:31075–85.
12. Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998; 12:1453–63.
13. Osborn MT, Chambers TC. Role of the stress-activated/c-Jun NH2-terminal protein kinase pathway in the cellular response to adriamycin and other chemotherapeutic drugs. J Biol Chem. 1996; 271:30950–5.

14. Cripe LD, Gelfanov VM, Smith EA, Spigel DR, Phillips CA, Gabig TG, et al. Role for c-jun N-terminal kinase in treatment-refractory acute myeloid leukemia (AML): signaling to multidrug-efflux and hyperproliferation. Leukemia. 2002; 16:799–812.

15. Lamy T, Goasguen JE, Mordelet E, Grulois I, Dauriac C, Drenou B, et al. P-glycoprotein (P-170) and CD34 expression in adult acute myeloid leukemia (AML). Leukemia. 1994; 8:1879–83.
16. Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnel TS, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1 and LRP in acute myeloid leukemia: a Southewest Oncology Group Study. Blood. 1999; 94:1086–99.
17. Van den Heuvel-Eibrink MM, van der Holt B, te Boekhorst PA, Pieters R, Schoester M, Lowenberg B, et al. MDR1 expression in an independent prognostic factor for response and survival in de novo acute myeloid leukemia. Br J Hematol. 1997; 99:76–83.
18. Filipits M Suchomel RW, Zochbauer S, Brunner R, Lechner K, Pirker R. Multidrug resistance associated protein in caute myeloid leukemia: No impact on treatment outcome. Clin Cancer Res. 1997; 3:1419–25.
19. Lunghi P, Tabilio A, Pinelli S, Valmadre G, Ridolo E, Albertini R, et al. Expression and activation of SHC/MAP kinase pathway in primary acute myeloid leukemia blasts. Hematol J. 2001; 2:70–80.

20. Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998; 92:2322–33.
21. Beck J, Handgretinger R, Dopfer R, Klingebiel T, Niethammer D, Gekeler V. Expression of mdr1, mrp, topoisomerase II alpha/beta, and cyclin A in primary or relapsed states of acute lymphoblastic leukaemias. Br J Haematol. 1995; 89:356–63.
22. Stammler G, Sauerbrey A, Volm M. Determination of DNA topoisomerase II in newly diagnosed childhood acute lymphoblastic leukemia by immunocytochemistry and RT-PCR. Cancer Lett. 1994; 84:141–7.

23. Vogler WR, Velez-Garcia E, Weiner RS, Flaum MA, Bartolucci AA, Omura GA, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: a Southeastern Cancer Study Group Study. J Clin Oncol. 1992; 10:1103–11.

24. Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990; 8:813–9.

25. Schaich M, Harbich-Brutscher E, Pascheberg U, Mohr B, Soucek S, Ehninger G, et al. Association of specific cytogenetic aberrations with mdr1 gene expression in acute myeloid leukemia and its implication for treatment outcome. Haematologica. 2002; 87:455–64.
26. Beck J, Handgretinger R, Klingebiel T, Dopfer R, Schaich M, Ehninger G, et al. Expression of PKC isozyme and MDR-associated genes in primary and relapsed state AML. Leukemia. 1996; 10:426–33.
Fig. 1.
RT-PCR results for mdr1, mrp1, gst π, and β2-microglobulin mRNA (internal control) with KG1 and CCRF-CEM T-cell lines as mdr1 mRNA positive and negative control and normal bone marrow (A). Expression levels of mdr1, mrp1 and gstπ mRNA using RT-PCR in patients with acute myeloid leukemia (B).
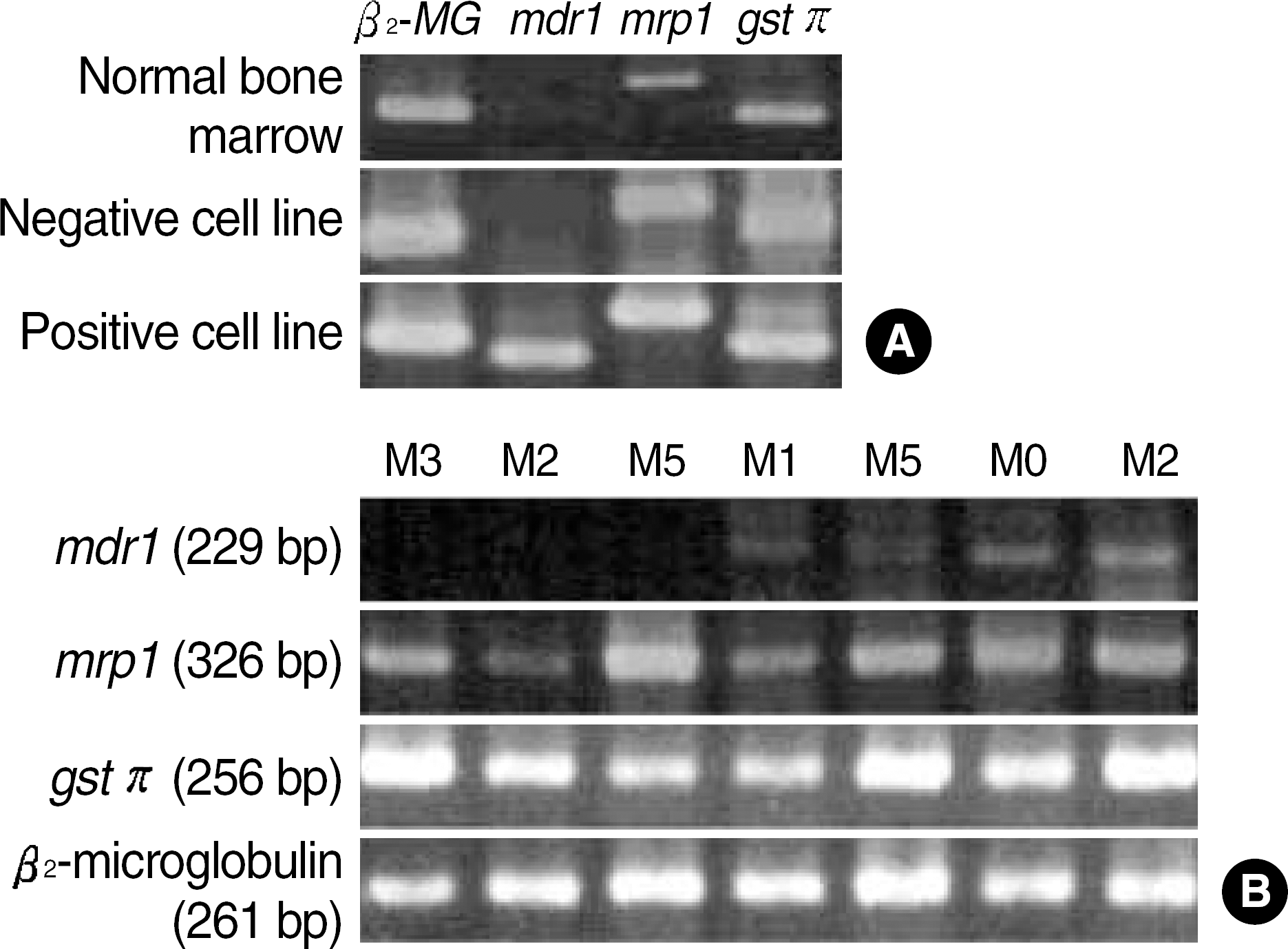
Fig. 2.
Immunoblotting of cell lysates of AML patients to analyse c-Jun-N-terminal kinase (JNK) activity and β-actin expression, and JNK activity (phospho c-Jun). JNK activity was undetectable in leukemic samples except 2 cases (middle). P, K562; N, normal bone marrow. Total JNK, 46, 54 kDa; phospho c-Jun, 35 kDa; β-actin; 42 kDa.
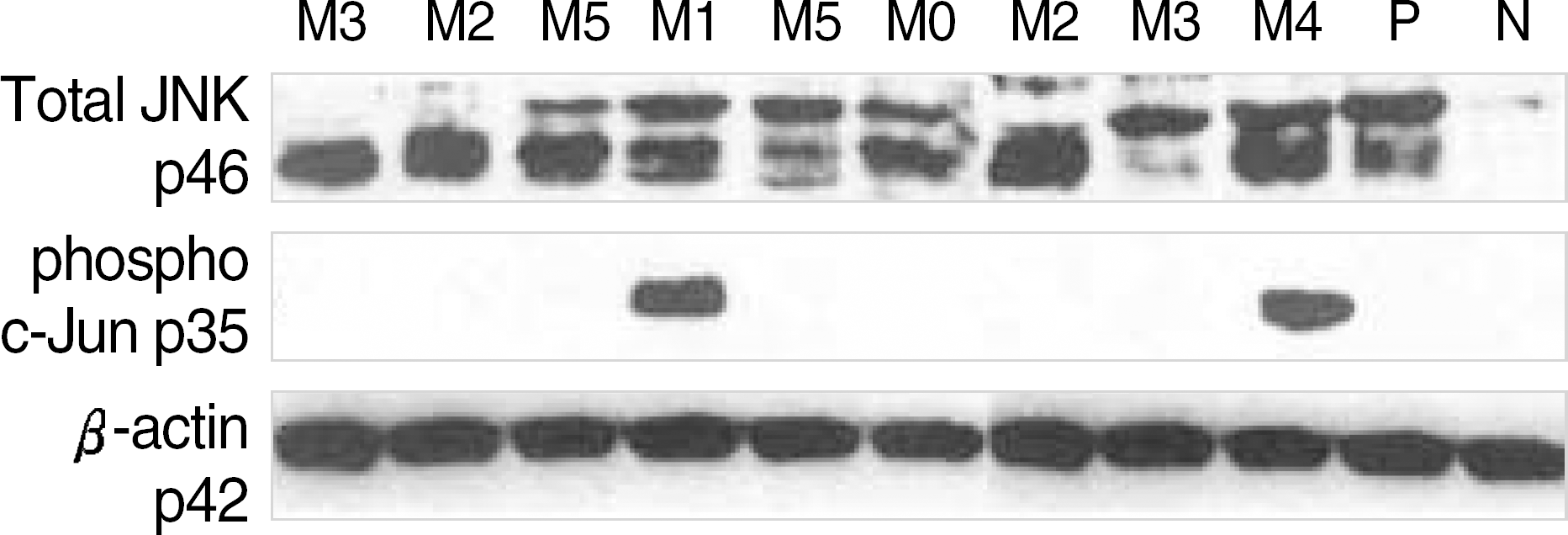
Table 1.
Clinical and laboratory parameters of patients with acute myeloid leukemia
| Parameters | |
|---|---|
| Male/female (number) | 26/26 |
| Age (yr) | 44.0* (17–68)† |
| Hemoglobin (g/dL) | 8.0 (3.9–15.0) |
| WBC (×109/L) | 32.2 (0.8–206) |
| Platelets (×109/L) | 42.0 (8.0–201.0) |
| Blasts in blood (%) | 62.5 (3.0–97.0) |
| FAB classification | |
| M0 | 3 |
| M1 | 16 |
| M2 | 13 |
| M3 | 11 |
| M4 | 6 |
| M5 | 3 |
| Chromosome abnormality‡ | |
| Favorable | 21 |
| Intermediate | 26 |
| Unfavorable | 5 |
| Treatment response | |
| CR (%) | 63.5 (33/52) |
Table 2.
Correlation among mdr1, mrp1 and gst π mRNA expression
| mrp1 | gstπ | |
|---|---|---|
| mdr1 | 0.651 (0.000)* | −0.031 (0.839) |
| mrp1 | 0.071 (0.641) |
Table 3.
Mdr1, mrp1, gstπ mRNA expression and clinical and laboratory findings in patients with acute myeloid leukemia
| Parameters | mdr1+ (%) | P | mrp1+ (%) | P | gst π+ (%) | P |
|---|---|---|---|---|---|---|
| Positive N | 20/52 (38.5) | 25/52 (48.1) | 25/46 (54.3) | |||
| Age (yrs) | ||||||
| <55 | 11/36 (30.6) | (0.122) | 14/36 (38.9) | (0.071) | 18/33 (54.5) | (1.000) |
| 55 | 9/16 (56.3) | 11/16 (68.8) | 7/13 (53.8) | |||
| Sex | ||||||
| Male | 11/26 (42.3) | 11/26 (42.3) | (0.579) | 15/23 (65.2) | (0.236) | |
| Female | 9/26 (34.6) | 14/26 (53.8) | 10/23 (43.5) | |||
| WBC (/L) | ||||||
| <5 × 109 | 11/28 (39.3) | (0.776) | 11/28 (39.3) | (0.254) | 14/23 (60.9) | (0.762) |
| ≥5 × 109 | 9/22 (40.9) | 13/22 (59.1) | 11/21 (52.4) | |||
| CD34 (%) | ||||||
| <20 | 8/22 (36.4) | (1.000) | 13/22 (59.1) | (0.578) | 10/22 (45.5) | (0.375) |
| ≥20 | 12/30 (40.0) | 12/30 (40.0) | 15/24 (62.5) | |||
| FAB | ||||||
| M0 | 2/3 (66.7) | (0.123) | 1/3 (33.3) | (0.154) | 2/3 (66.7) | (0.228) |
| M1 | 5/16 (31.3) | 9/16 (56.3) | 10/13 (76.9) | |||
| M2 | 8/13 (61.5) | 7/13 (53.8) | 5/11 (45.5) | |||
| M3 | 1/11 (9.1) | 2/11 (18.2) | 5/10 (50.0) | |||
| M4 | 3/5 (60.0) | 3/5 (60.0) | 3/6 (50.0) | |||
| M5 | 1/3 (33.3) | 3/3 (100) | 3/3 (100) | |||
| Chromosome | ||||||
| Good | 6/21 (28.6) | (0.105) | 7/21 (33.3) | (0.121) | 10/19 (52.6) | (0.469) |
| Intermediate | 10/26 (38.5) | 14/26 (53.8) | 11/22 (50.0) | |||
| Poor | 4/5 (80.0) | 4/5 (80.0) | 4/5 (80.0) | |||
| Treatment | ||||||
| Response | 7/33 (21.2) | (0.001*) | 12/33 (36.4) | (0.043*) | 15/29 (51.7) | (0.762) |
| Failure | 13/19 (68.4) | 13/19 (68.4) | 10/17 (58.8) |
Table 4.
Chemotherapeutic response and overall survivals of AML patients according to clinical findings, and mdr1, mrp1, gst π mRNA and JNK activity
| Parameters | N of cases |
Chemotherapy |
Overall survival |
||
|---|---|---|---|---|---|
| Remission (%) | P | Median±SE (months) | P | ||
| Age | |||||
| <55 | 36 | 27 (75.0) | 0.014* | 16.0±3.3 | 0.001* |
| ≥55 | 16 | 6 (37.5) | 2.0±3.0 | ||
| Sex | |||||
| Male | 26 | 15 (57.7) | 0.565 | 60.0±3.1 | 0.117 |
| Female | 26 | 18 (69.2) | 16.0±6.9 | ||
| WBC (/L) | |||||
| <5×109 | 28 | 23 (82.1) | 0.004* | 17.0±1.8 | 0.004* |
| ≥5× 109 | 22 | 9 (40.9) | 3.0±0.5 | ||
| CD34 (%) | |||||
| <20 | 24 | 14 (58.3) | 0.568 | 21.0±3.5 | 0.129 |
| ≥20 | 28 | 19 (67.9) | 7.0±4.0 | ||
| Chromosome | |||||
| Good | 21 | 19 (90.5) | 0.002* | 27.1±3.2 | 0.000* |
| Intermediate | 26 | 13 (50.0) | 8.3±1.6 | ||
| Poor | 5 | 1 (20.0) | 2.4±0.4 | ||
| mdr1 | |||||
| Negative | 32 | 26 (81.3) | 0.001* | 19.9±2.8 | 0.001* |
| Positive | 20 | 7 (35.0) | 2.0±0.7 | ||
| mp1 | |||||
| Negative | 27 | 21 (77.8) | 0.043* | 17.0±5.7 | 0.003* |
| Positive | 25 | 12 (48.0) | 4.0±1.6 | ||
| gst π | |||||
| Low | 21 | 14 (66.7) | 0.762 | 21.6±3.4 | 0.296 |
| High | 25 | 15 (60.0) | 7.0±3.6 | ||
| JNK activity | |||||
| Negative | 28 | 20 (71.4) | 0.103 | 16.0±8.8 | 0.013* |
| Positive | 2 | 0 (0.0) | 2.0±0.0 | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download