Abstract
Background
Fecal occult blood tests (FOBTs) have been widely used as a means of colorectal cancer screening. Automated FOBTs using immunologic principles have the advantages such as quantitation, high specificity, and high throughput. We evaluated a newly-introduced automated FOBT analyzer, OC-SENSOR neo (OC neo) (Eiken Chemical Co., Japan).
Methods
The precision, linearity, and carry-over rate of OC neo were assessed with specimens prepared in accordance with the guidelines of CLSI. We performed a parallel test between OC neo and OC-SENSOR I (OC I) (Eiken Chemical Co.) using 300 consecutive stool specimens and 60 OC I-positive specimens. The results were analyzed with SPSS version 13.0 (SPSS Inc., USA).
Results
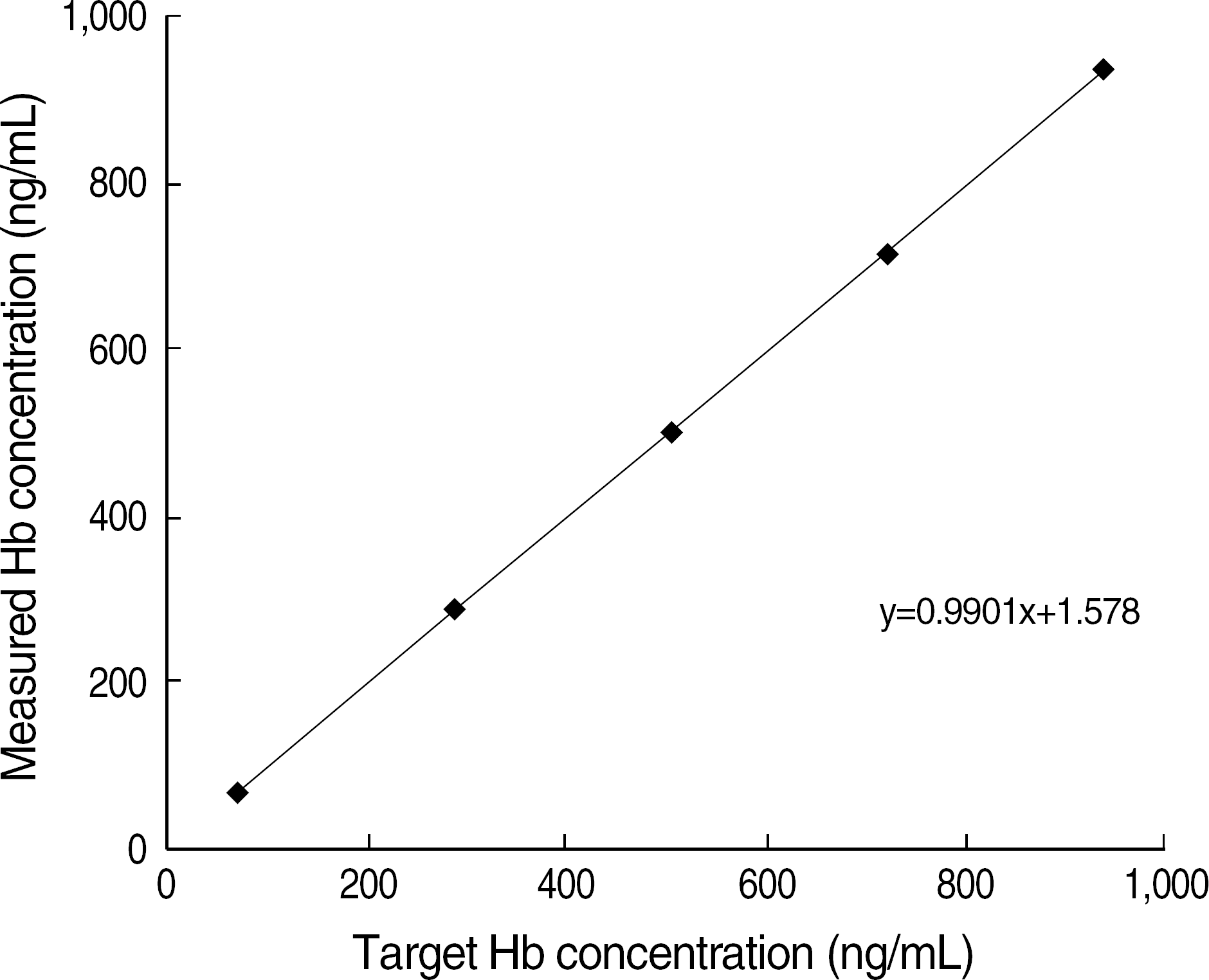
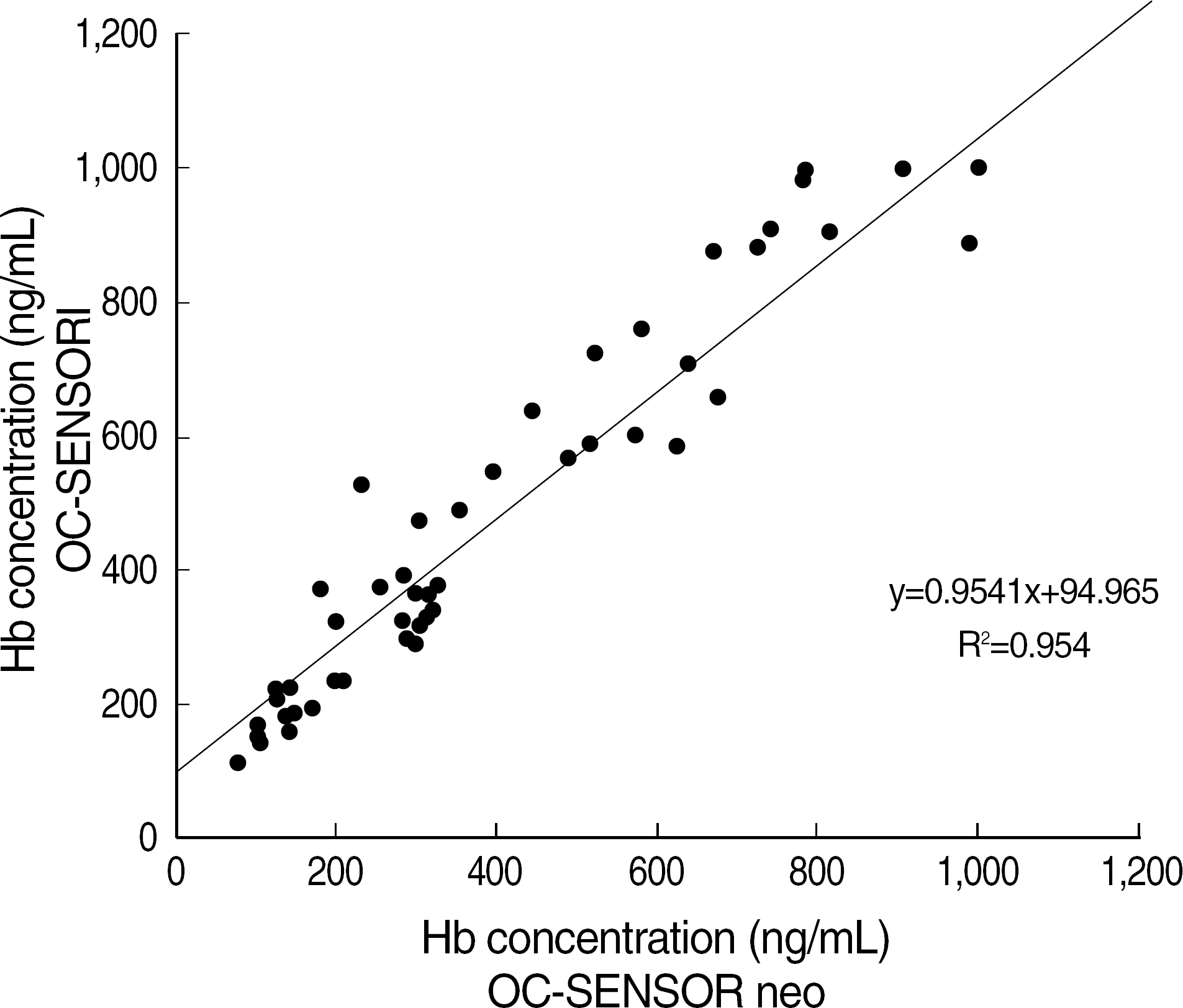
The coefficients of variation (CV) of within-run, between-run, and between-day using OC-Control L (Eiken Chemical Co.) of ca. 150 ng/mL were 3.5–7.8%, 4.5–8.8% and 4.9–5.0%, respectively. The linear regression coefficient and carry-over rate with the range of 67.8–939.4 ng/mL were 0.9998 (P<0.001), and 0.1%, respectively. Correlation coefficient between OC neo and OC I was R2=0.954 (P<0.001) for 60 OC I-positive specimens. The positive and negative interpretations of 300 consecutive specimens by OC neo were completely consistent with those of OC I.
References
1. Kim DW, Bang YJ, Heo DS, Kim NK. Colorectal cancer in Korea: characteristics and trends. Tumori. 2002; 88:262–5.

2. Helm J, Choi J, Sutphen R, Barthel JS, Albrecht TL, Chirikos TN. Current and evolving strategies for colorectal cancer screening. Cancer Control. 2003; 10:193–204.

4. Bromer MQ, Weinberg DS. Screening for colorectal cancer-now and the near future. Semin Oncol. 2005; 32:3–10.

5. Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997; 112:594–642.

6. Suggested technique for fecal occult blood testing and interpretation in colorectal cancer screening. American College of Physicians. Ann Intern Med. 1997; 126:808–10.
7. Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993; 328:1365–71.
8. Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996; 348:1472–7.

9. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996; 348:1467–71.

10. Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000; 343:1603–7.

11. Steinmetz J, Spyckerelle Y, Gueguen R, Dupre C. Colorectal cancer screening in Health Examination Centers. Gastroenterol Clin Biol. 2006; 30:832–7.

12. Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA. 2003; 289:1288–96.
14. Han WS, Choi YM, Lee DH. Comparison of guaiac method with latex method for the fecal occult blood test. Korean J Clin Pathol. 1996; 16:318–23.
15. Vilkin A, Rozen P, Levi Z, Waked A, Maoz E, Birkenfeld S, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005; 100:2519–25.

16. Horiuchi Y, Masubuchi J, Oikawa S, Matsuda R, Hishinuma A, Ieiri T. Evaluation of fully automated fecal occult blood analyzer OC-SENSOR neo and OC-SENSOR II. Jap Assoc Clin Lab Auto. 2003; 28:40–6.
17. Touzuka S, Aoyama K, Kamino T. Fundamental study on fully automated fecal occult blood immunochemical analyzer, OC-SENSOR neo. Med Exam Equip-Reag. 2002; 25:121–5.
18. National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative measurement procedures; A statistical approach; Approved guideline. Document EP6-A. Vilanova, PA: National Commitee for Clinical Laboratory Standards;2003.
19. National Committee for Clinical Laboratory Standards. Evaluation of precision performance of quantitative measurement methods; Approved guideline-Second edition. Document EP5-A2.Vilanova, PA: National Commitee for Clinical Laboratory Standards;2004.
20. Pesce MA. Laboratory antomation. In. Kaplan LA, Pesce AJ, editors. Clinical Chemistry. 4th ed.Baltimore: Mosby;2003. :294.
21. Hong SB, Kim HS, Park HS, Lee DH. Evaluation of the HM-JACK Automatic Analyzer for Fecal Occult Blood Test. J Lab Med Qual Assur. 2002; 24:221–4.
22. Kim DC, Cho SS, Song J, Kim EC, Kim JQ. Evaluation of the OC-SENSOR for Immunological Fecal Occult Blood Test. Korean J Clin Pathol Qual Control. 1998; 20:281–8.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download