Abstract
Background
We noticed an abrupt increase in the isolation of Stenotrophomonas maltophilia from bronchoalveolar lavage (BAL) specimens collected at Chosun University Hospital. We performed surveillance cultures in order to identify the source of what appeared to be a pseudo-outbreak.
Methods
To investigate a possible nosocomial outbreak of S. maltophilia, we performed culture of 11 environmental specimens obtained from a bronchoscopy room and two bronchoscopes. Pulsed-field gel electrophoresis (PFGE) was used to examine the genetic relatedness among the strains of S. maltophilia recovered from BAL specimens of 3 patients and 1 environmental sample, as well as 9 unrelated strains of S. maltophilia as a control.
Results
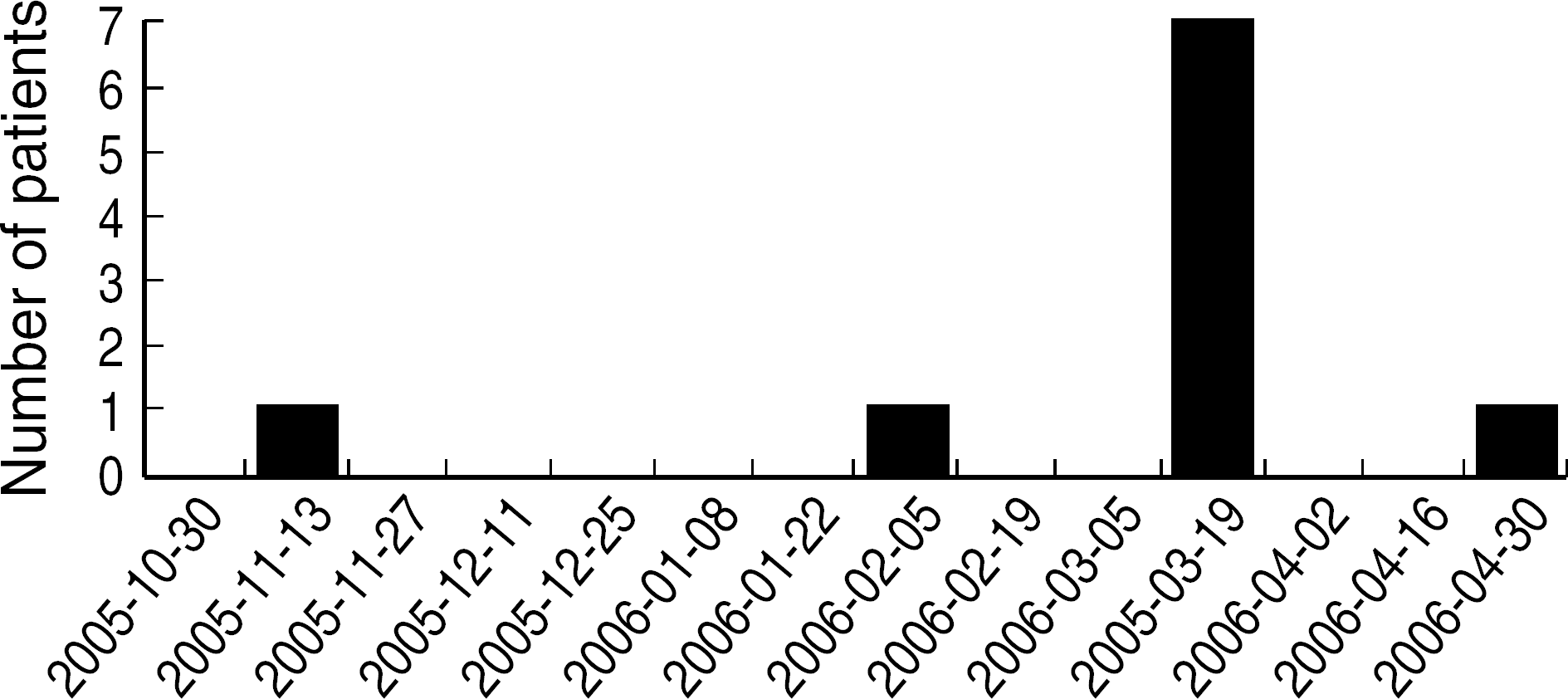
During a 7 day-period in March 2006, S. maltophilia was isolated from the BAL specimens of 7 of 13 (54%) patients, compared to only 5 of 188 (2.6%) patients during the 6-month period prior to that period. S. maltophilia was isolated from 1 of the 11 environmental samples, which was obtained from a fiberoptic bronchoscope suction channel. All 7 patient isolates and one environmental isolate exhibited similar antibiotic susceptibility patterns. PFGE analysis of the genomic DNA from epidemic strains demonstrated an identical banding pattern, whereas each of epidemiologically unrelated strains showed a unique electrophoretic pattern.
Conclusions
Apparently one of the hospital bronchoscopes became contaminated with S. maltophilia during a bronchoscopic procedure. It is likely that subsequent specimen contamination occurred because the bronchoscope had been inadequately cleaned and disinfected. The pseudo-outbreak was controlled successfully by removing the source of infection.
Go to : 
References
1. Sakhnini E, Weissmann A, Oren I. Fulminant Stenotrophomonas maltophilia soft tissue infection in immunocompromised patients: an outbreak transmitted via tap water. Am J Med Sci. 2002; 323:269–72.
2. Vartivarian S, Anaissie E, Bodey G, Sprigg H, Rolston K. A changing pattern of susceptibility of Xanthomonas maltophilia to antimicrobial agents: implication for therapy. Antimicrob Agents Chemother. 1994; 38:624–7.
3. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Sixteenth Informational supplement, M100-S16 (M2). Wayne, PA: Clinical and Laboratory Standards Institute;2006.
4. Valdezate S, Vindel A, Martin-Davila P, Del Saz BS, Baquero F, Canton R. High genetic diversity among Stenotrophomonas maltophilia strains despite their originating at a single hospital. J Clin Microbiol. 2004; 42:693–9.
5. Morrison AJ Jr, Hoffmann KK, Wenzel RP. Associated mortality and clinical characteristics of nosocomial Pseudomonas maltophilia in a university hospital. J Clin Microbiol. 1986; 24:52–5.
6. Muder RR, Yu VL, Dummer JS, Vinson C, Lumish RM. Infections caused by Pseudomonas maltophilia. Expanding clinical spectrum. Arch Intern Med. 1987; 147:1672–4.
7. Jones RN, Sader HS, Beach ML. Contemporary in vitro spectrum of activity summary for antimicrobial agents tested against 18569 strains non-fermentative Gram-negative bacilli isolated in the SENTRY Antimicrobial Surveillance Program (1991–2001). Int J Antimicrob Agents. 2003; 22:551–6.
9. Cetre JC, Nicolle MC, Salord H, Perol M, Tigaud S, David G, et al. Outbreaks of contaminated broncho-alveolar lavage related to intrinsically defective bronchoscopes. J Hosp Infect. 2005; 61:39–45.
10. Singh N, Belen O, Leger MM, Campos JM. Cluster of Trichosporon mucoides in children associated with a faulty bronchoscope. Pediatr Infect Dis J. 2003; 22:609–12.
11. Weber DJ, Rutala WA. Lessons from outbreaks associated with bronchoscopy. Infect Control Hosp Epidemiol. 2001; 22:403–8.

12. Wang HC, Liaw YS, Yang PC, Kuo SH, Luh KT. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibre-optic bronchoscope suction channel. Eur Respir J. 1995; 8:1259–62.
13. Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993; 118:117–28.

15. Walter VA, DiMarino AJ Jr. American society for gastrointestinal endoscopy-society of gastroenterology nurses and associates endoscope reprocessing guidelines. Gastrointest Endosc Clin N Am. 2000; 10:265–73.

16. Park ES, Kim OS, Kim KM, Kim YS, Jeong SY, Yoon SW. Descriptive study for status of usage of disinfectants in Korea. Korean J Nosocomial Infect Control. 2001; 6:17–32.
Go to : 
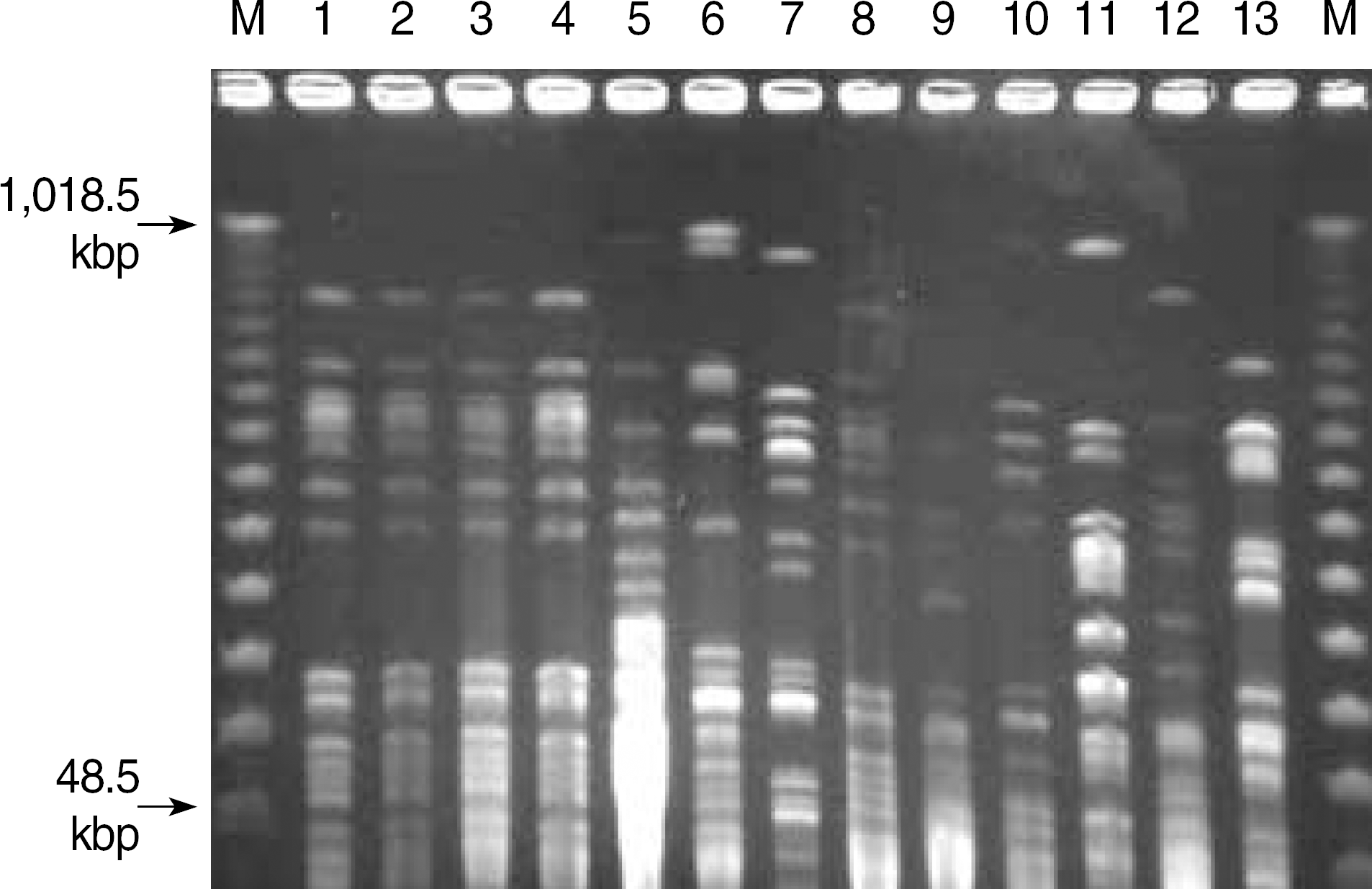 | Fig. 2.PFGE analysis of S. maltophilia isolates. Chromosomal DNA was digested with XbaI. Lane M, DNA molecular marker; lane 1 to 3, pseudo-outbreak isolates; lane 4, environmental isolate; lane 5 to 13, non-outbreak isolates. |
Table 1.
Clinical features exhibited by the 7 patients whose bronchoalveolar lavage specimens yielded S. maltophilia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download