Abstract
Background
Ischemic stroke is a complex condition influenced by many factors. Previous studies have demonstrated that inflammatory markers might play a role in such vascular diseases. Therefore the purpose of this study was to compare the expression of inflammatory markers in Korean ischemic stroke patients and to investigate their relationship to APOE polymorphism.
Methods
The patient group consisted of 275 patients with large artery atherosclerosis (LAA, n= 169) and small artery occlusion (SAO, n=106). One hundred and nineteen age matched healthy subjects were recruited as the control group. Serum levels of three inflammatory markers (matrix metal-loproteinase, MMP-9; tissue inhibitor of metalloproteinase-1, TIMP-1; and high-sensitivity C-reactive protein, hs-CRP) were measured in each patient by using commercially available kits. Comparison of clinical risk factors, inflammatory marker levels, and APOE genotypes between the stroke patient group and control group and between the two patient subgroups was assessed.
Results
Comparison of the stroke group to control group showed significantly elevated levels of circulating MMP-9 (P<0.01) and hs-CRP (P=0.01). Comparison between the individual subgroups revealed a significantly higher level of only TIMP-1 in the LAA subgroup compared to the SAO subgroup (P<0.01). There was no significant difference in inflammatory marker levels among each allele carrier.
Conclusions
The present study revealed the obvious tendency of increased circulating inflammatory markers in the patients with acute ischemic attack, especially MMP-9 and hs-CRP. Our observations suggest that measurement of serum MMP-9, TIMP-1, and hs-CRP levels may be useful in the diagnosis of ischemic stroke patients.
References
1. Carr FJ, McBride MW, Carswell HV, Graham D, Strhorn P, Clark JS, et al. Genetic aspects of stroke: human and experimental studies. J Cereb Blood Flow Metab. 2002; 22:767–73.

2. Kim JS, Han SR, Chung SW, Kim BS, Lee KS, Kim YI, et al. The apolipoprotein E epsilon4 haplotype is an important predictor for recurrence in ischemic cerebrovascular disease. J Neurol Sci. 2003; 206:31–7.
3. MacLeod MJ, De Lange RP, Breen G, Meiklejohn D, Lemmon H, Clair DS. Lack of association between apolipoprotein E genotype and ischemic stroke in a Scottish population. Eur J Clin Invest. 2001; 31:570–3.
4. Elbaz A, Amarenco P. Genetic susceptibility and ischaemic stroke. Curr Opin Neurol. 1999; 12:47–55.

5. Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lacet Neurol. 2004; 3:227–35.

6. Kolovou GD, Daskalova DCh, Hatzivassiliou M, Yiannakouris N, Pilatis ND, Elisaf M, et al. The epsilon 2 and 4 alleles of apolipoprotein E and ischemic vascular events in the Greek population-implications for the interpretation of similar studies. Angiology. 2003; 54:51–8.
7. Grocott HP, Newman MF, El-Moalem H, Bainbridge D, Butler A, Laskowitz DT. Apolipoprotein E genotype differentially influences the proinflammatory and anti-inflammatory response to cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001; 122:622–3.

8. Yoshida H, Hasty AH, Major AS, Ishiguro H, Su YR, Gleaves LA, et al. Isoform-specific effects of apolipoprotein E on atherogenesis: gene transduction studies in mice. Circulation. 2001; 104:2820–5.
9. Murphy MM, Vilella E, Ceruelo S, Figuera L, Sanchez M, Camps J, et al. The MTHFR C677T, APOE, and PON55 gene polymorphisms show relevant interactions with cardiovascular risk factors. Clin Chem. 2002; 48:372–5.
10. Kim JS, Han SR, Chung SW, Kim KS, Kim JW, Kim BS. Apolipoprotein E4 genotype in patients with ischemic cerebrovascular disease in Korea: a preliminary study. J Korean Neurol Assoc. 2001; 19:19–23.
11. Kang SY, Lee WI. Apolipoprotein E polymorphism in ischemic stroke patients with different pathogenic origins. Korean J Lab Med. 2006; 26:210–6.
12. Adams HP Jr, Woolson RF, Biller J, Clarke W. Studies of Org 10172 in patients with acute ischemic stroke. TOAST Study Group. Haemostasis. 1992; 22:99–103.
13. Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004; 35:57–63.

14. Kouwenhoven M, Carlstrom C, Ozenci V, Link H. Matrix metalloproteinase and cytokine profiles in monocytes over the course of stroke. J Clin Immunol. 2001; 21:365–75.
15. Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998; 56:149–71.

16. Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001; 32:1759–66.
17. Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003; 34:40–6.

18. Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003; 34:2165–70.

19. Orbe J, Fernandez L, Rodriguez JA, Rabago G, Belzunce M, Monasterio A, et al. Different expression of MMPs/TIMP-1 in human atherosclerotic lesions. Relation to plaque features and vascular bed. Atherosclerosis. 2003; 170:269–76.

20. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001; 103:1813–8.

21. Sellmayer A, Limmert T, Hoffmann U. High sensitivity C-reactive protein in cardiovascular risk assessment. CRP mania or useful screening? Int Angiol. 2003; 22:15–23.
22. Leu HB, Lin CP, Lin WT, Wu TC, Chen JW. Risk stratification and prognostic implication of plasma biomarkers in nondiabetic patients with stable coronary artery disease: the role of high sensitivity C-reactive protein. Chest. 2004; 126:1032–9.
23. Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relationship of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003; 167:73–9.
24. Di Napoli M, Papa F, Villa Pini Stroke Data Bank Investigators. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke. 2002; 33:1763–71.
Fig. 1.
Inflammatory marker levels between ε4/non-ε4 carriers and ε2/non-ε2 carriers in ischemic stroke group and control group.
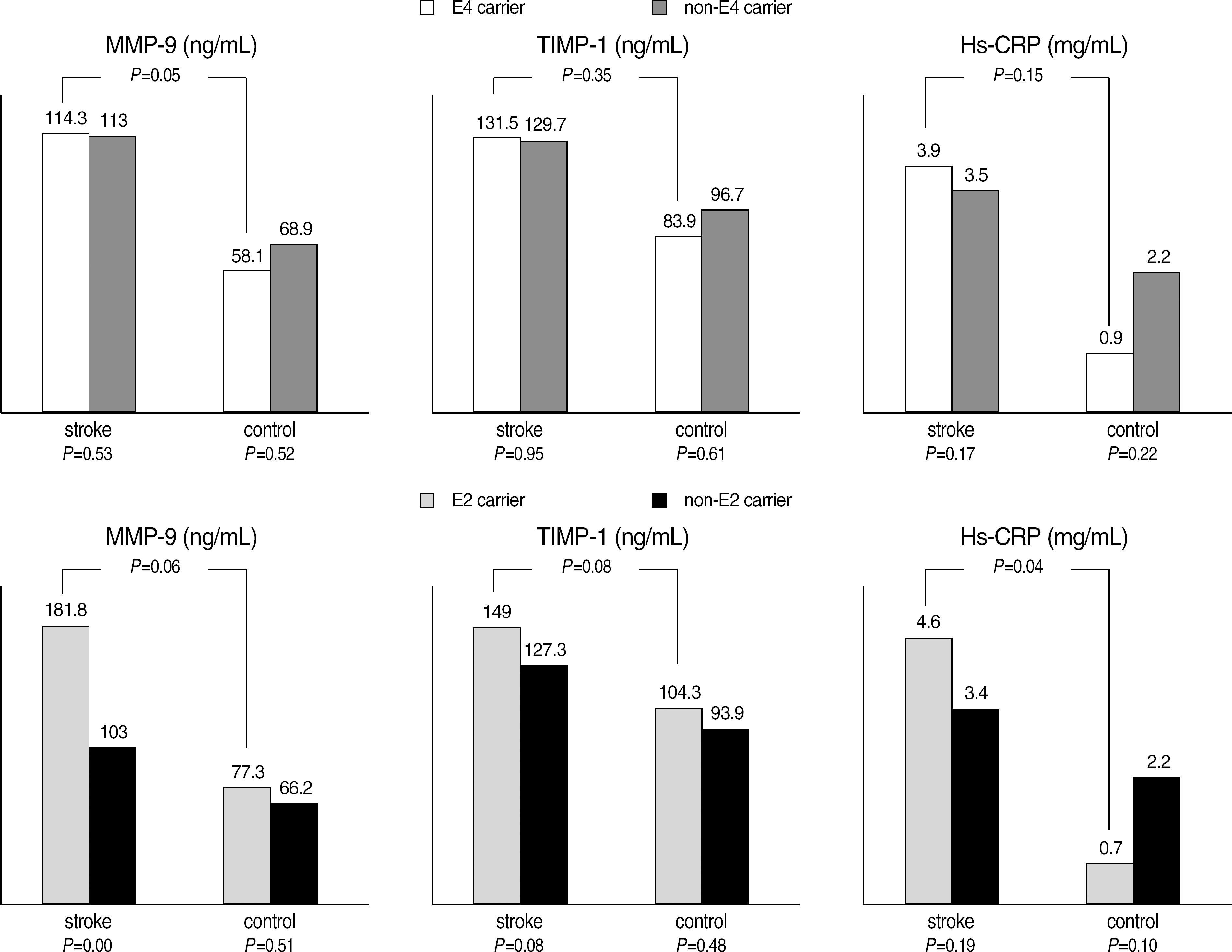
Fig. 2.
Inflammatory marker levels between ε4/non-ε4 carriers and ε2/non-ε2 carriers in large artery group and small artery group.
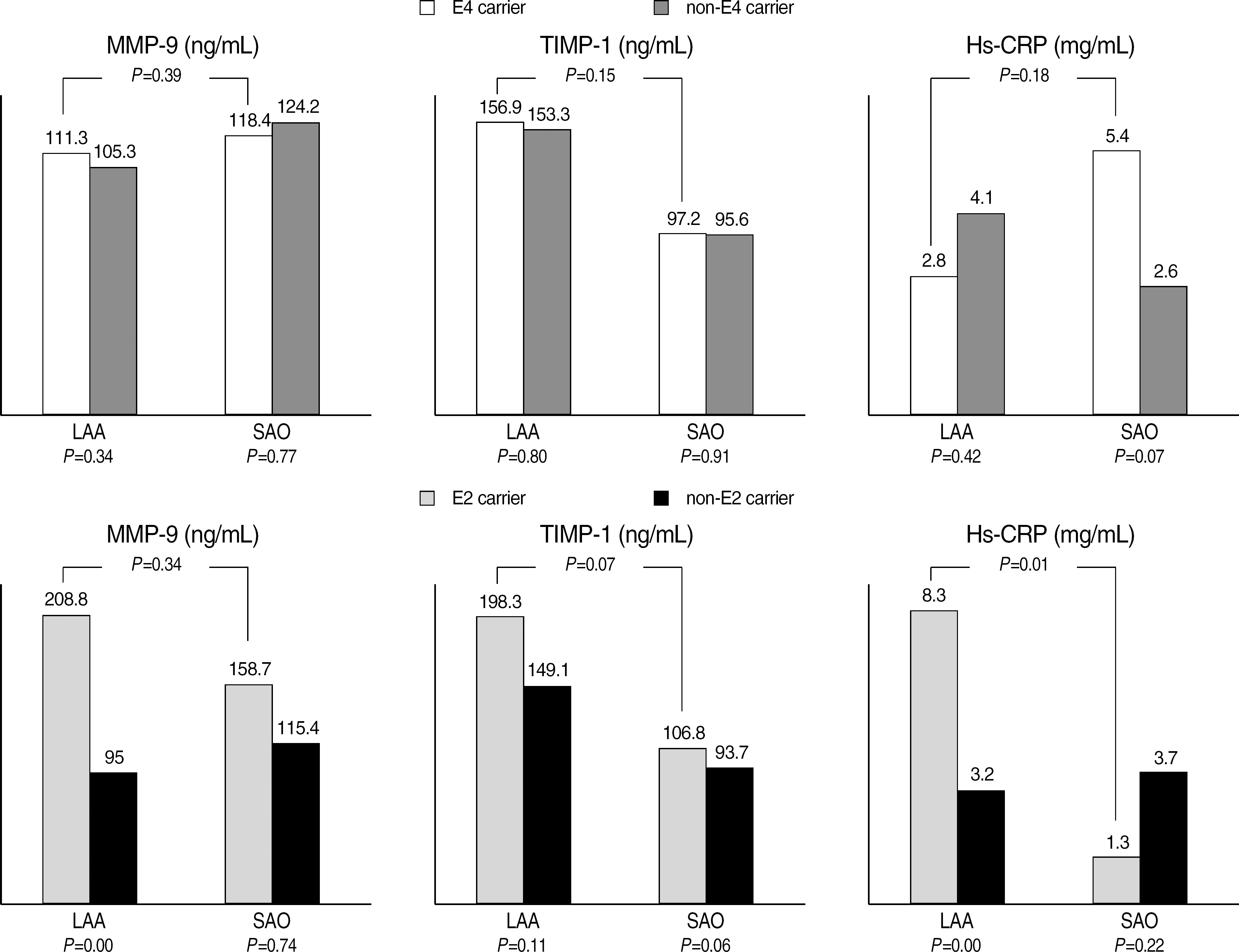
Table 1.
Distribution of clinical characteristics and risk factors in ischemic stroke group and control group
| Characteristics |
Ischemic stroke group |
Control group | P† | |||
|---|---|---|---|---|---|---|
| LAA | SAO | P* | Total | |||
| Number | 169 | 106 | 275 | 119 | ||
| Sex (M:F) | 113:56 | 68:38 | 0.64 | 181:94 | 62:57 | 0.01 |
| Age | 63.3 (±8.9) | 61.2 (±9.0) | 0.12 | 62.5 (±8.9) | 61.5 (±7.5) | 0.08 |
| Smoking history | 23 (13.6%) | 9 (8.5%) | 0.21 | 32 (11.6%) | 20 (16.8%) | 0.13 |
| Hypertension | 109 (64.5%) | 68 (64.2%) | 0.96 | 177 (64.3%) | ||
| DM | 47 (27.8%) | 24 (22.6%) | 0.36 | 71 (25.8%) | ||
| Previous stroke history | 17 (10.0%) | 19 (17.9%) | 0.06 | 36 (13.0%) | ||
| IHD history | 7 (4.1%) | 8 (7.5%) | 0.22 | 15 (5.5%) | ||
Table 2.
Expression of inflammatory marker levels and apoE polymorphism in ischemic stroke group and control group
| Inflammatory marker |
Ischemic stroke group |
Control group | P† | |||
|---|---|---|---|---|---|---|
| LAA | SAO | P* | Total | |||
| Number | 169 | 106 | 275 | 119 | ||
| MMP-9 (ng/mL) | 101.15 (±123.16) | 114.64 (±106.15) | 0.55 | 106.35 (±116.88) | 68.64 (±68.33) | <0.01 |
| TIMP-1 (ng/mL) | 151.29 (±88.15) | 90.89 (±45.45) | <0.01 | 128.01 (±80.14) | 93.12 (±49.14) | 0.06 |
| hs-CRP (mg/dL) | 0.40 (±0.85) | 0.32 (±0.81) | 0.66 | 0.37 (±0.83) | 0.20 (±0.45) | 0.01 |
Table 3.
Comparison of inflammatory markers between ε2/non-ε2 carriers and ε4/non-ε4 carriers in stroke cases and control group
|
ε2 allele |
ε4 allele |
|||||
|---|---|---|---|---|---|---|
| ε2 carrier | non-ε2 carrier | P* | ε4 carrier | non-ε4 carrier | P† | |
| MMP-9 (ng/mL) | ||||||
| Stroke | 181.8 (±215.8) | 103.0 (±106.2) | <0.01 | 114.3 (±138.2) | 113.0 (±125.2) | 0.53 |
| Control | 77.3 (±78.1) | 66.2 (±65.0) | 0.51 | 58.1 (±49.0) | 68.9 (±68.6) | 0.52 |
| P‡ | 0.06 | 0.05 | ||||
| TIMP-1 (ng/mL) | ||||||
| Stroke | 149.0 (±106.0) | 127.3 (±82.1) | 0.08 | 131.5 (±82.4) | 129.7 (±86.8) | 0.95 |
| Control | 104.3 (±44.7) | 93.9 (±49.8) | 0.48 | 83.9 (±43.7) | 96.7 (±49.8) | 0.61 |
| P‡ | 0.08 | 0.35 | ||||
| Hs-CRP (mg/dL) | ||||||
| Stroke | 0.46 (±1.07) | 0.34 (±0.75) | 0.19 | 0.39 (±1.18) | 0.35 (±0.64) | 0.17 |
| Control | 0.07 (±0.56) | 0.22 (±0.49) | 0.10 | 0.09 (±0.13) | 0.22 (±0.49) | 0.22 |
| P‡ | 0.04 | 0.15 | ||||
Table 4.
Comparison of inflammatory markers between ε4/non-ε4 carriers and ε2/non-ε2 carriers in large artery and small artery group
|
ε2 allele |
ε4 allele |
|||||
|---|---|---|---|---|---|---|
| ε2 carrier | non-ε2 carrier | P* | ε4 carrier | non-ε4 carrier | P† | |
| MMP-9 (ng/mL) | ||||||
| LAA | 208.8 (±272.7) | 95.0 (±110.2) | <0.01 | 111.3 (±156.2) | 105.3 (±133.4) | 0.34 |
| SAO | 158.7 (±159.2) | 115.4 (±99.3) | 0.74 | 118.4 (±113.2) | 124.2 (±112.2) | 0.77 |
| P‡ | 0.34 | 0.39 | ||||
| TIMP-1 (ng/mL) | ||||||
| LAA | 198.3 (±125.8) | 149.1 (±93.1) | 0.11 | 156.9 (±92.1) | 153.3 (±99.5) | 0.80 |
| SAO | 106.8 (±63.8) | 93.7 (±44.0) | 0.06 | 97.2 (±51.5) | 95.6 (±46.9) | 0.91 |
| P‡ | 0.07 | 0.15 | ||||
| hs-CRP (mg/dL) | ||||||
| LAA | 0.83 (±1.52) | 0.32 (±0.61) | <0.01 | 0.28 (±0.67) | 0.41 (±0.78) | 0.42 |
| SAO | 0.13 (±0.14) | 0.37 (±0.94) | 0.22 | 0.54 (±1.65) | 0.26 (±0.33) | 0.07 |
| P‡ | 0.01 | 0.18 | ||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download