Abstract
Background
Oral anticoagulation with warfarin requires routine monitoring of prothrombin time to maintain the international normalized ratio (INR) within the appropriate therapeutic range. CoaguChek XS (Roche Diagnositic, Germany) is a portable coagulometer that measures the INR. We evaluated the precision and accuracy of CoaguCheck XS by comparing it with CA-1500 (Sysmex, Japan).
Methods
We analyzed the CV and the correlation of all INR results measured in 68 samples obtained from patients treated with warfarin and 10 samples from control subjects with no history of anticoagulant therapy with CoaguChek XS and CA-1500. We compared the turn-around time between two instruments and evaluated the differences between the results obtained with venous and capillary blood samples and those obtained with different lots of the test strip. We also evaluated the precision of the two instruments in 5 repeated tests with samples of normal and increased INR.
Results
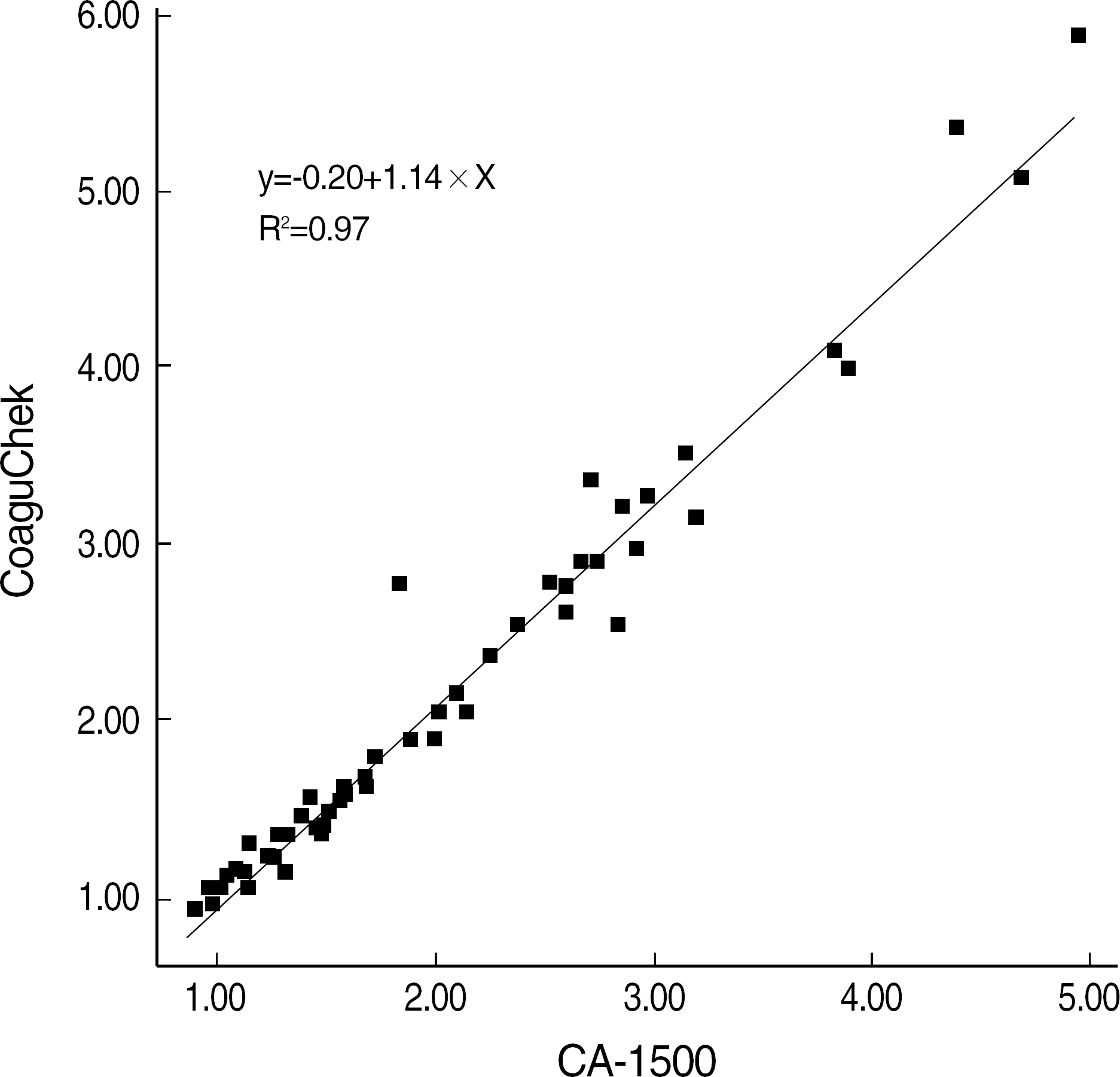
Mean INR values of 5 repeated tests with the same samples were similar. The correlation of INR values between two instruments was excellent (r2=0.97, P=0.001), and the difference in the values between the two instruments was mostly within the 95% limit of agreement, but was shown to increase in direct proportion to INR values. The turn-around time of CoaguChek XS was shorter than that of CA-1500. The differences between venous and capillary blood and between different lots of the test trip were not significant (P>0.05).
Go to : 
References
1. Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996; 348:423–8.
2. Palareti G, Manotti C, DAngelo A, Pengo V, Erba N, Moia M, et al. Thrombotic events during oral anticoagulant treatment: results of the inception-cohort, prospective, collaborative ISCOAT study: IS-COAT study group (Italian Study on Complications of Oral Anticoagulant Therapy). Thromb Haemost. 1997; 78:1438–43.
3. Linder MW. Genetic mechanisms for hypersensitivity and resistance to the anticoagulant Warfarin. Clin chim Acta. 2001; 308:9–15.

4. Loebstein R, Yonath H, Peleg D, Almog S, Rotenberg M, Lubetsky A, et al. Interindividual variability in sensitivity to warfarin–Nature or nurture? Clin Pharmacol Ther. 2001; 70:159–64.

5. Sawicki PT. A structured teaching and self-management program for patients receiving oral anticoagulation: a randomized controlled trial. Working Group for the Study of Patient Self-Management of Oral Anticoagulation. JAMA. 1999; 281:145–50.
6. Horstkotte D, Piper C, Wiemer M. Optimal Frequency of Patient Monitoring and Intensity of Oral Anticoagulation Therapy in Valvular Heart Disease. J Thromb Thrombolysis. 1998; 5:19–24.
7. Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995; 333:11–7.

8. Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126:204–33.
9. Clinical and Laboratory Standards Institute. Collection, transport, and processing of blood specimen for testing plasma-based coagulation assays; approved guideline H21-A4,. 4th ed.Wayne, PA: Clinical and Laboratory Standards Institute;2003.
10. Douketis JD, Lane A, Milne J, Ginsberg JS. Accuracy of a portable International Normalization Ratio monitor in outpatients receiving long-term oral anticoagulant therapy: comparison with a laboratory reference standard using clinically relevant criteria for agreement. Thromb Res. 1998; 92:11–7.
11. Marzinotto V, Monagle P, Chan A, Adams M, Massicotte P, Leaker M, et al. Capillary whole blood monitoring of oral anticoagulants in children in outpatient clinics and the home setting. Pediatr Cardiol. 2000; 21:347–52.

12. Cosmi B, Palareti G, Moia M, Carpenedo M, Pengo V, Biasiolo A, et al. Accuracy of a portable prothrombin time monitor (Coagu-check) in patients on chronic oral anticoagulant therapy: a prospective multicenter study. Thromb Res. 2000; 100:279–86.
Go to : 
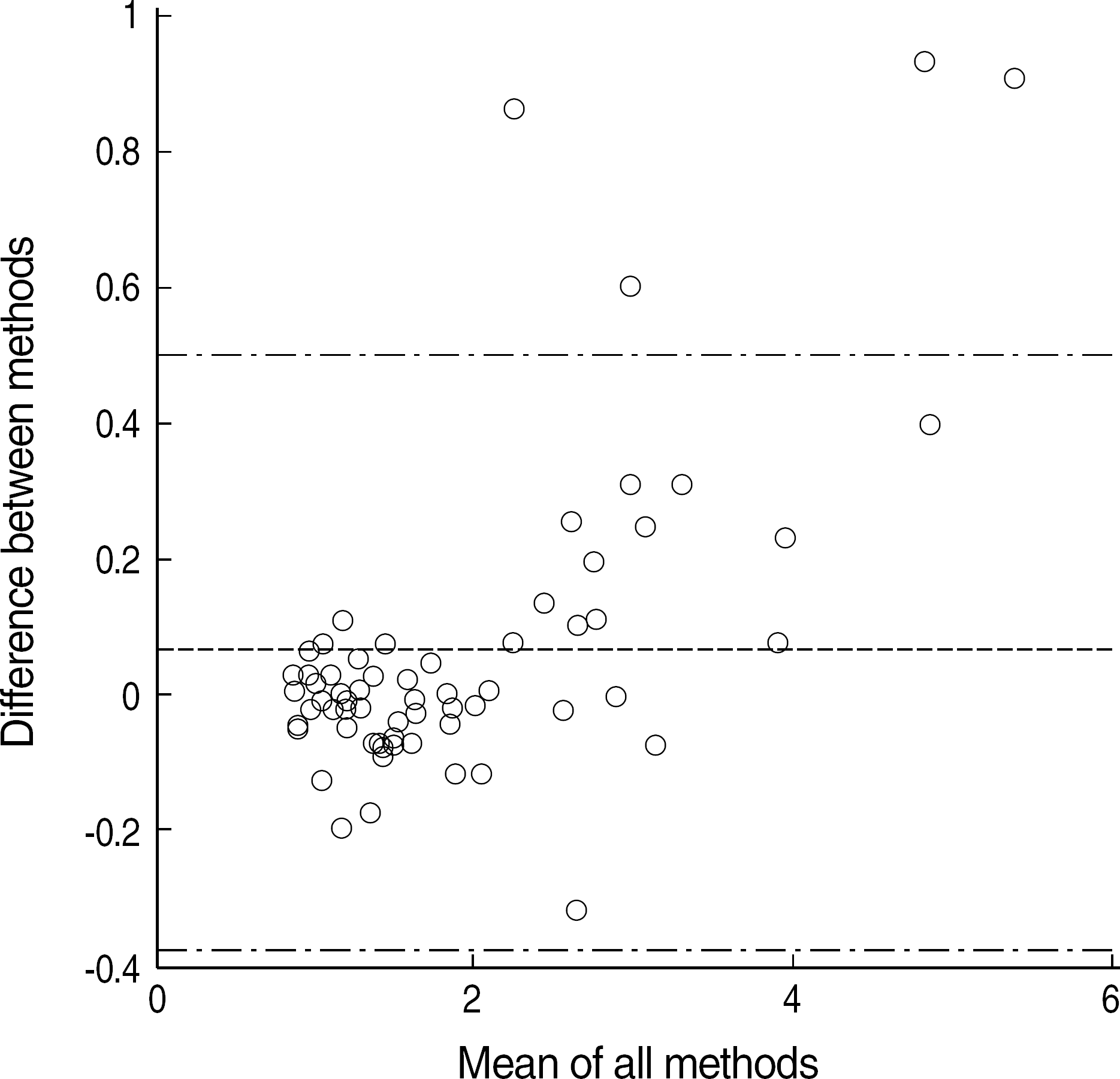 | Fig. 2.Bias plot shows most of the value of difference between two methods are within the 95% limit of agreement (between upper and lower chain line). |
Table 1.
Comparison of PT results of samples with normal and increased INR by 5 repeated tests using two instruments
|
Normal INR sample |
Increased INR sample |
|||
|---|---|---|---|---|
| CoaguChek XS | CA-1500 | CoaguChek XS | CA-1500 | |
| Mean | 0.99 | 0.96 | 3.16 | 2.95 |
| SD | 0.03 | 0.01 | 0.08 | 0.02 |
| CV | 3.19 | 0.59 | 2.67 | 0.53 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download