Abstract
Background
We intended to find the mutations of von Willebrand factor (VWF) gene as the most important contributing factor of von Willebrand disease (VWD) in Korean patients.
Methods
In 40 known vWD patients mutations of vWF gene were sought by direct sequencing of PCR products targeting exons 18, 19, 20, 26, 28 and 52 frequently implicated as the locations of mutation. For factors other than VWF gene contributing to VWD phenotype, we tested ABO blood group and measured ADAMTS13 activity in VWD patients.
Results
Twenty-seven cases (67.5%) were type 1 vWD, 3 cases (7.5%) type 3, and 5 cases (12.5 %) type 2A. Three cases were type 2A or 2B (7.5%) and 2 cases were suspected to be type 2N (5.0 %). Among them six candidate missense mutations were found: V1279I, R1306W, R1308C, and V1316M were previously reported in type 2B and type 1 vWD, and C858W and T1477I were novel findings. All patients were heterozygotes. Blood group O was overly represented in VWD patients, while ADAMTS13 activity of the patients was not significantly different from that of normal control.
Go to : 
References
1. Ruggeri ZM, Ware J, editors. Von Willebrand factor. Loscalzo J. Schafer AI, editor. Thrombosis and hemorrhage. 3rd ed.Philadelphia: Lippincott Williams & Wilkins;2003. p. 246–53.
2. Ginsburg D, Sadler JE. von Willebrand disease: a database of point mutations, insertions, and deletions. For the Consortium on von Willebrand Factor Mutations and Polymorphisms, and the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1993; 69:177–84.
3. Eikenboom JC, Reitsma PH, Peerlinck KM, Briet E. Recessive inheritance of von Willebrand's disease type I. Lancet. 1993; 341:982–6.

4. Kroner PA, Foster PA, Fahs SA, Montgomery RR. The defective interaction between von Willebrand factor and factor VIII in a patient with type 1 von Willebrand disease is caused by substitution of Arg19 and His54 in mature von Willebrand factor. Blood. 1996; 87:1013–21.

5. Eikenboom JC, Matsushita T, Reitsma PH, Tuley EA, Castaman G, Briet E, et al. Dominant type 1 von Willebrand disease caused by mutated cysteine residues in the D3 domain of von Willebrand factor. Blood. 1996; 88:2433–41.

6. Eikenboom JC, Castaman G, Vos HL, Bertina RM, Rodeghiero F. Characterization of the genetic defects in recessive type 1 and type 3 von Willebrand disease patients of Italian origin. Thromb Haemost. 1998; 79:709–17.

7. Casana P, Martinez F, Haya S, Espinos C, Aznar JA. Association of the 3467C>T mutation (T1156M) in the von Willebrand's factor gene with dominant type 1 von Willebrand's disease. Ann Hematol. 2001; 80:381–3.
8. O'Brien LA, James PD, Othman M, Berber E, Cameron C, Notley CR, et al. Founder von Willebrand factor haplotype associated with type 1 von Willebrand disease. Blood. 2003; 102:549–57.
9. Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987; 69:1691–5.

10. Song KS, Kim HK, Park YS. Detection of Novel C4517G (Ser743Trp) Mutation in a Family with Type 2A von Willebrand Disease. Korean J Hematol. 2003; 38:274–8.
11. Mancuso DJ, Tuley EA, Westfield LA, Lester-Mancuso TL, Le Beau MM, Sorace JM, et al. Human von Willebrand factor gene and pseudogene: structural analysis and differentiation by polymerase chain reaction. Biochemistry. 1991; 30:253–69.

12. Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VW-F73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005; 129:93–100.

13. Hagiwara T, Inaba H, Yoshida S, Nagaizumi K, Arai M, Hanabusa H, et al. A novel mutation Gly 1672–>Arg in type 2A and a homozygous mutation in type 2B von Willebrand disease. Thromb Haemost. 1996; 76:253–7.
14. Cooney KA, Nichols WC, Bruck ME, Bahou WF, Shapiro AD, Bowie EJ, et al. The molecular defect in type IIB von Willebrand disease. Identification of four potential missense mutations within the putative GpIb binding domain. J Clin Invest. 1991; 87:1227–33.

15. Donner M, Andersson AM, Kristoffersson AC, Nilsson IM, Dahlback B, Holmberg L. An Arg545—-Cys545 substitution mutation of the von Willebrand factor in type IIB von Willebrand's disease. Eur J Haematol. 1991; 47:342–5.
16. Murray EW, Giles AR, Bridge PJ, Peake IR, Lillicrap DP. Cosegregation of von Willebrand factor gene polymorphisms and possible germinal mosaicism in type IIB von Willebrand disease. Blood. 1991; 77:1476–83.

17. Randi AM, Rabinowitz I, Mancuso DJ, Mannucci PM, Sadler JE. Molecular basis of von Willebrand disease type IIB. Candidate mutations cluster in one disulfide loop between proposed platelet glycoprotein Ib binding sequences. J Clin Invest. 1991; 87:1220–6.

18. Casana P, Martinez F, Espinos C, Haya S, Lorenzo JI, Aznar JA. Search for mutations in a segment of the exon 28 of the human von Willebrand factor gene: new mutations, R1315C and R1341W, associated with type 2M and 2B variants. Am J Hematol. 1998; 59:57–63.
19. Bowen DJ, Collins PW. An amino acid polymorphism in von Willebrand factor correlates with increased susceptibility to proteolysis by ADAMTS13. Blood. 2004; 103:941–7.

20. Bowen DJ, Collins PW, Lester W, Cumming AM, Keeney S, Grundy P, et al. The prevalence of the cysteine1584 variant of von Willebrand factor is increased in type 1 von Willebrand disease: co-segregation with increased susceptibility to ADAMTS13 proteolysis but not clinical phenotype. Br J Haematol. 2005; 128:830–6.

21. Miller CH, Haff E, Platt SJ, Rawlins P, Drews CD, Dilley AB, et al. Measurement of von Willebrand factor activity: relative effects of ABO blood type and race. J Thromb Haemost. 2003; 1:2191–7.

22. Choi SY, Kim SI, Cho HI. Study on gene frequencies of blood groups in Koreans. Korean J Hematol. 1984; 19:63–75.
23. Kroner PA, Friedman KD, Fahs SA, Scott JP, Montgomery RR. Abnormal binding of factor VIII is linked with the substitution of glutamine for arginine 91 in von Willebrand factor in a variant form of von Willebrand disease. J Biol Chem. 1991; 266:19146–9.

24. Jorieux S, Tuley EA, Gaucher C, Mazurier C, Sadler JE. The mutation Arg (53)—-Trp causes von Willebrand disease Normandy by abolishing binding to factor VIII. Studies with recombinant von Willebrand factor. Blood. 1992; 79:563–7.

25. Mazurier C, Meyer D. Factor VIII binding assay of von Willebrand factor and the diagnosis of type 2N von Willebrand disease–results of an international survey. On behalf of the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1996; 76:270–4.
26. Meyer D, Fressinaud E, Gaucher C, Lavergne JM, Hilbert L, Ribba AS, et al. Gene defects in 150 unrelated French cases with type 2 von Willebrand disease: from the patient to the gene. INSERM Network on Molecular Abnormalities in von Willebrand Disease. Thromb Haemost. 1997; 78:451–6.
27. Vossen CY, Hasstedt SJ, Rosendaal FR, Callas PW, Bauer KA, Broze GJ, et al. Heritability of plasma concentrations of clotting factors and measures of a prethrombotic state in a protein C-deficient family. J Thromb Haemost. 2004; 2:242–7.

28. Castaman G, Eikenboom JC, Bertina RM, Rodeghiero F. Inconsistency of association between type 1 von Willebrand disease phenotype and genotype in families identified in an epidemiological investigation. Thromb Haemost. 1999; 82:1065–70.

29. Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003; 1:33–40.

30. Castaman G, Eikenboom JC, Bertina RM, Rodeghiero F. Inconsistency of association between type 1 von Willebrand disease phenotype and genotype in families identified in an epidemiological investigation. Thromb Haemost. 1999; 82:1065–70.

31. Souto JC, Almasy L, Soria JM, Buil A, Stone W, Lathrop M, et al. Genome-wide linkage analysis of von Willebrand factor plasma levels: results from the GAIT project. Thromb Haemost. 2003; 89:468–74.

32. Mannucci PM, Capoferri C, Canciani MT. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol. 2004; 126:213–8.

33. Harvey PJ, Keightley AM, Lam YM, Cameron C, Lillicrap D. A single nucleotide polymorphism at nucleotide – 1793 in the von Willebrand factor (VWF) regulatory region is associated with plasma VWF: Ag levels. Br J Haematol. 2000; 109:349–53.
Go to : 
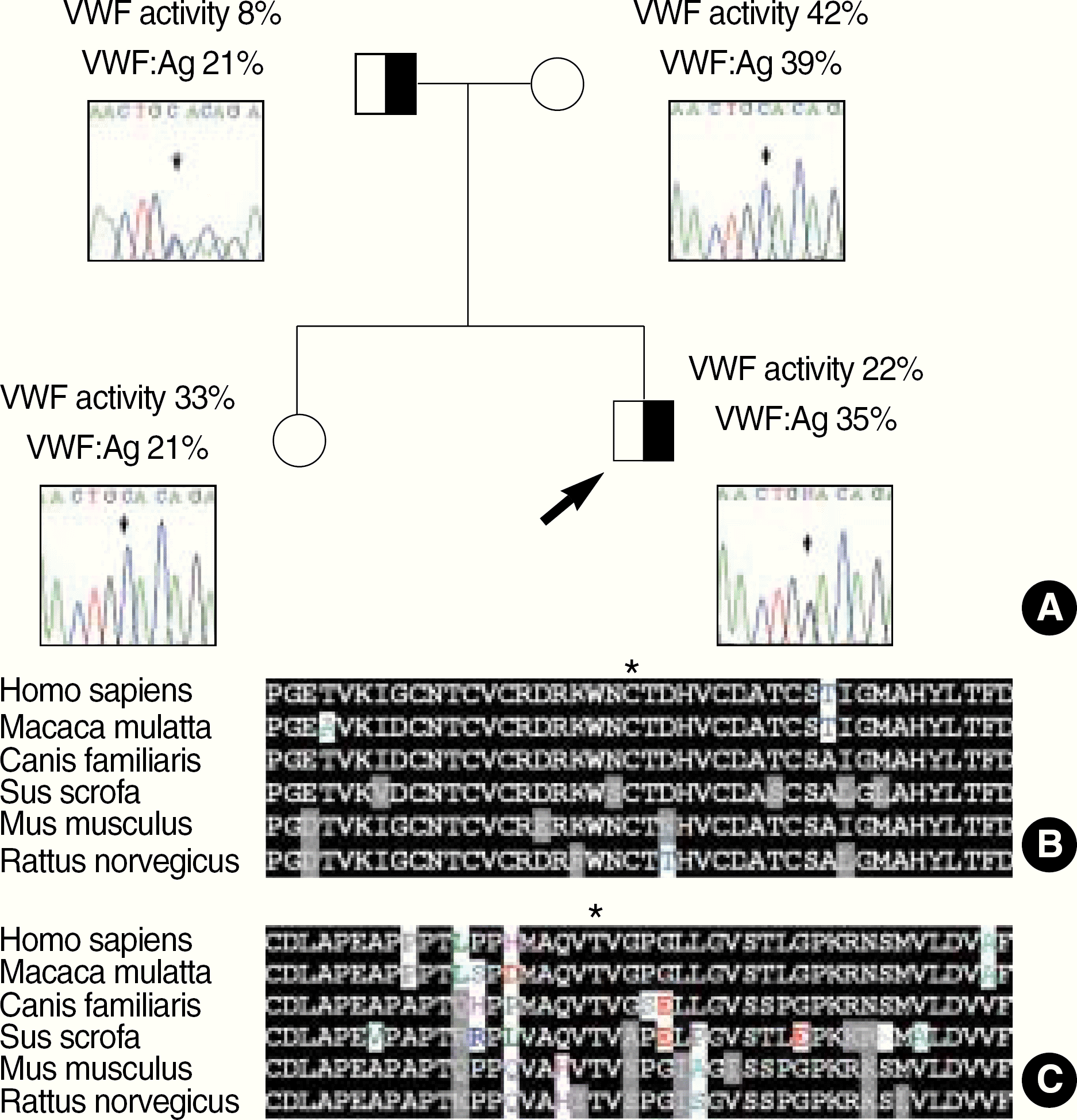 | Fig. 1.The probable role of the novel findings C858W and T1477I in causing VWD. The C858W segregated with decreased VWF activity within the family of the patient 37, while mildly decreased VWF: Ag was observed in all members of the family (A). The residues 858C and 1477T (indicated by asterisk) within VWF are more strictly preserved throughout several different mammalian species than other immediate neighboring residues, implying its essentiality for wholeness of VWF in regard to its synthesis, stability or function (B, C). |
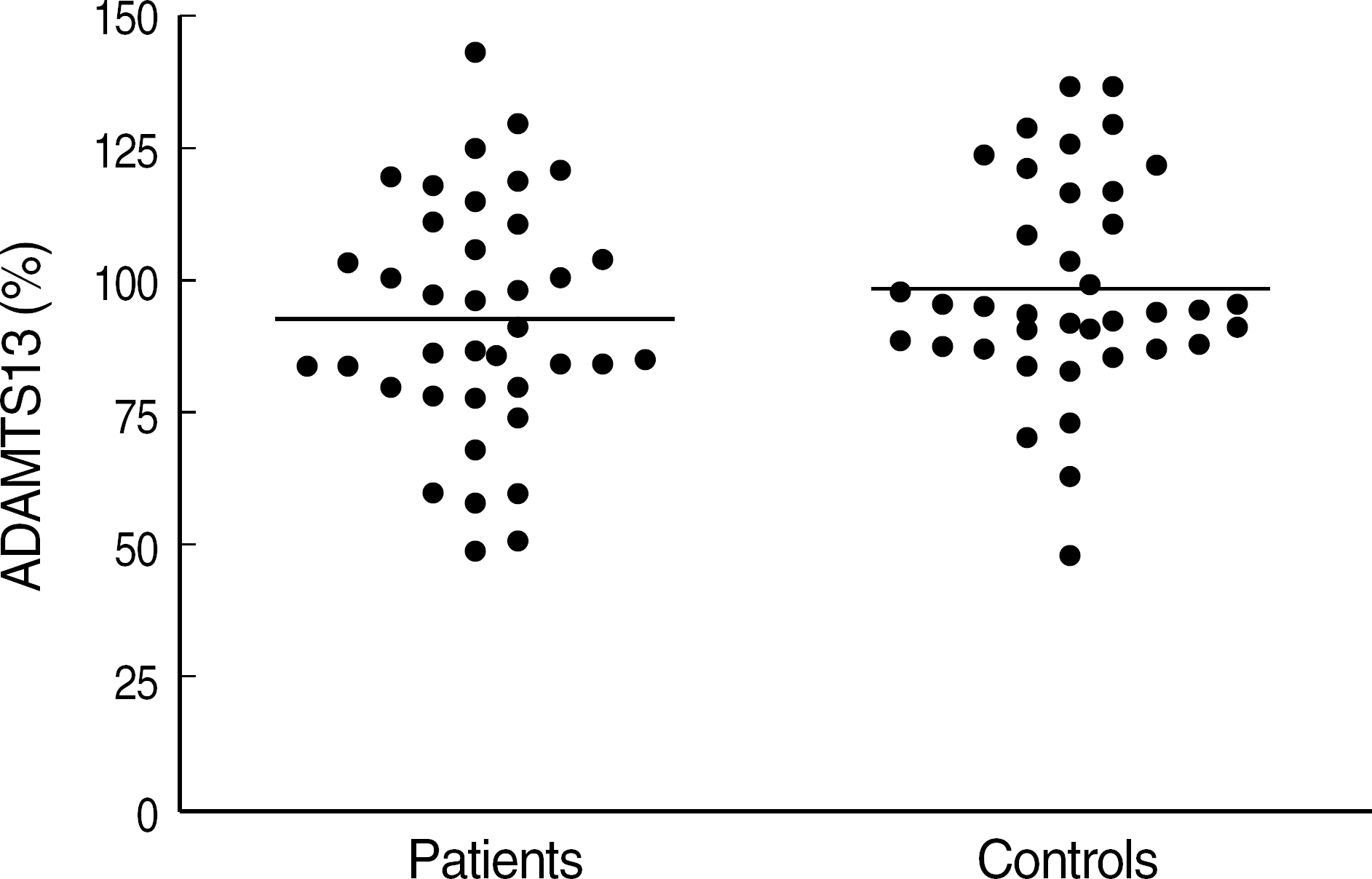 | Fig. 2.ADAMTS13 activity in VWD patients compared with normal controls. There was no significant difference between ADAMTS13 activity between VWD patients (n=38) and normal healthy control (n=38). |
Table 1.
Number of each subtype among the VWD patients included and positive results of the mutation screening
Table 2.
Laboratory data of the patients for whom candidate implicated mutations of VWF gene were found and past reports of each mutation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download