Abstract
Background
The urea breath test (UBT) is regarded as a highly reliable, noninvasive tool for diagnosing Helicobacter pylori infection. We compared a recently developed low-dose 38 mg 13C-urea capsule, which is able to eliminate oral urease effects and does not require positional changes during the test, with the conventionally used 100 mg 13C-urea tablet method.
Methods
Thirty-nine volunteers were tested under informed consent with both 13C-UBT methods, Helifinder® and UBiT-IR300®, with a minimum 2-week washout period. The pre-ingestion and 20-minute post-ingestion breath samples were analyzed with an isotope ratio mass spectrometer for Helifinder, and a nondispersive isotope-selective infrared spectrophotometer for UBiT samples.
Go to : 
References
1. Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994; 330:1267–71.
2. The Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993; 341:1359–62.
3. National Institute of Health Consensus conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994; 272:65–9.
4. Graham DY, Klein PD, Evans DJ Jr, Evans DG, Alpert LC, Opekun AR, et al. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987; 1:1174–7.
5. Slomianski A, Schubert T, Cutler AF. [13C]Urea breath test to confirm eradication of Helicobacter pylori. Am J Gastroenterol. 1995; 90:224–6.
6. European Helicobacter pylori Study Group (EHPSG). Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997; 41:8–13.
7. Savarino V, Mela GS, Zentilin P, Bisso G, Pivari M, Mansi C, et al. Comparison of isotope ratio mass spectrometry and nondispersive isotope-selective infrared spectroscopy for 13C-urea breath test. Am J Gastroenterol. 1999; 94:1203–8.
8. Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut. 1999; 45(S1):):I18–22.
9. Parente F, Bianchi Porro G. The (13)C-urea breath test for noninvasive diagnosis of Helicobacter pylori infection: which procedure and which measuring equipment? Eur J Gastroenterol Hepatol. 2001; 13:803–6.
10. Ohara S, Kato M, Saito M, Fukuda S, Kato C, Hamada S, et al. Comparison between a new 13C-urea breath test, using a film-coated tablet, and the conventional 13C-urea breath test for the detection of Helicobacter pylori infection. J Gastroenterol. 2004; 39:621–8.
11. Kato M, Saito M, Fukuda S, Kato C, Obara S, Hamada S, et al. 13C-urea breath test, using a new compact nondispersive isotope-selective infrared spectrophotometer: comparison with mass spectrometry. J Gastroenterol. 2004; 39:629–34.
12. Kim YS, Chun HJ, Jeen YT, Kim HK, Hyun JH, Chung IS, et al. The efficacy of 38 mg low dose capsule-based 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Korean J Gastrointest Endosc. 2005; 30:126–32.
Go to : 
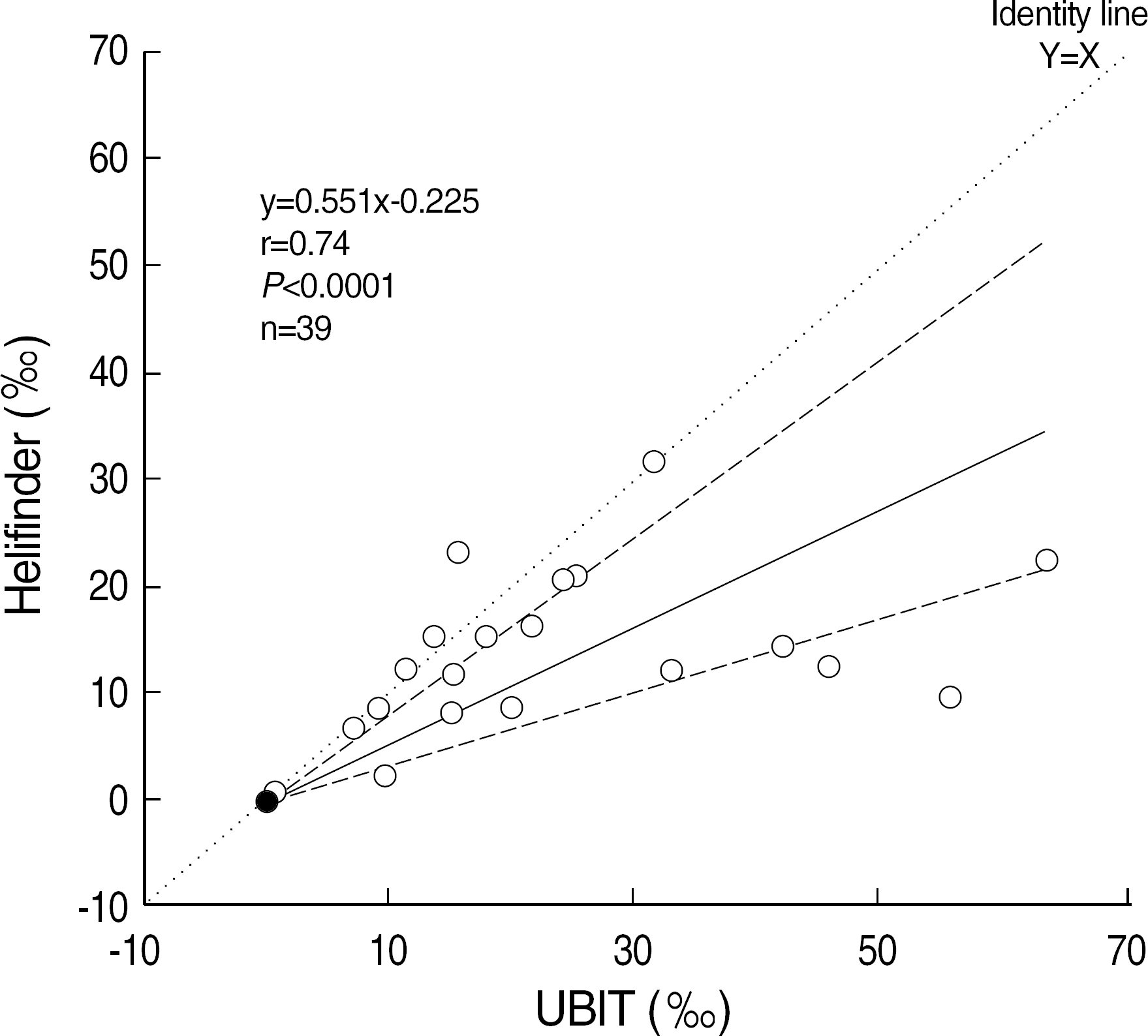 | Fig. 1.Passing and Bablok comparison of Helifinder® and UBiT® 13C values (‰). Dashed lines show the 95% confidence limits of the regression line. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download